Summary: Volume decreases in cortical areas, the amygdala, and basal forebrain in Parkinson’s patients correlated with worsening symptoms of the disease.
Source: Human Brain Project
A team of researchers from Forschungszentrum Jülich, Heinrich-Heine-University Düsseldorf and Ernst-von-Bergmann Klinikum Potsdam analyzed the changes of brain volumes in 37 Parkinson’s patients and 27 controls at up to 15 time points over up to 8.8 years using magnetic resonance imaging (MRI). Previous in vivo studies had either measured brain volumes in Parkinson’s patients only at fewer time points or over shorter periods.
At the beginning of the study, the team found that the volumes of several brain regions were smaller in the Parkinson’s disease patients than in the control group, whereas some regions were enlarged in the brains of patients, presumably because of compensatory effects.
Over time, the difference between the groups became increasingly widespread and pronounced: The brain volumes of the Parkinson’s disease patients declined almost twice as quickly as those of the control group, particularly in the gray matter.
Mostly affected by this volume decrease were the temporal and occipital lobes, neighboring parts of the inferior parietal lobe and ventral parts of the frontal lobe.
The team analyzed in detail which parts of the brain changed over time using neuroanatomical atlases, primarily the Julich Brain Atlas, which is openly accessible via the HBP’s EBRAINS infrastructure.
This detailed anatomical analysis revealed a very specific regional pattern of volume changes in the Parkinson’s disease patients that was different from healthy aging.
The researchers found that the volume decreases of cortical areas, amygdala and basal forebrain in Parkinson’s patients were correlated with the worsening of clinical symptoms.
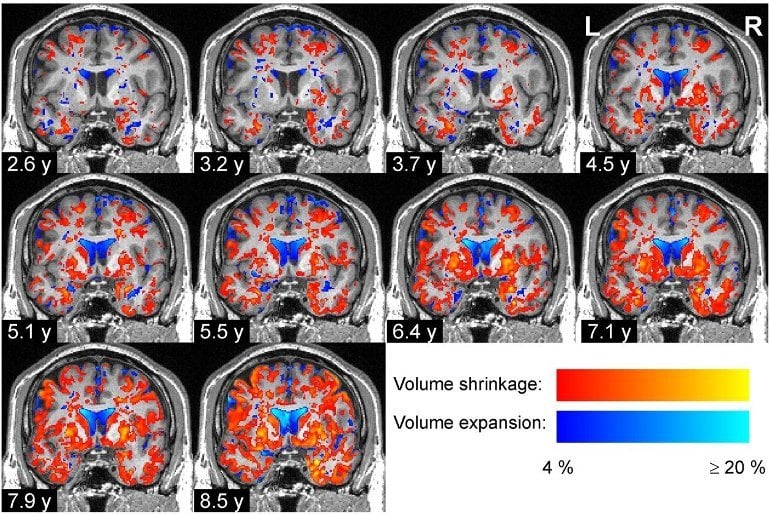
The specific pattern of regional volume decreases that they revealed coincided with the stage scheme of Parkinson’s disease proposed by Heiko Braak in 2003.
Braak and colleagues had analyzed 168 postmortem brains for the presence of aggregates form by misfolded alpha-synuclein proteins and postulated progressive propagation of the disease in six stages starting at the lower brain stem nuclei and olfactory bulb and progressing via the midbrain towards the prefrontal and premotor cortices.
“Our study provides detailed in vivo data about how the brain of Parkinson’s disease patients degenerates across space and time, and the pattern we find coincides with Braak’s stages,” says Peter Pieperhoff, first author of the recent study.
About this Parkinson’s disease research news
Author: Press Office
Source: Human Brain Project
Contact: Press Office – Human Brain Project
Image: The image is credited to Pieperhoff et al
Original Research: Open access.
“Regional changes of brain structure during progression of idiopathic Parkinson’s disease—A longitudinal study using deformation based morphometry” by Pieperhoff et al. Cortex
Abstract
Regional changes of brain structure during progression of idiopathic Parkinson’s disease—A longitudinal study using deformation based morphometry
Idiopathic Parkinson’s disease (PD) is a neurodegenerative disorder with a broad spectrum of motor and non-motor symptoms. The neuropathological characteristics of idiopathic PD are the degeneration of dopaminergic neurons in the striatum, and the propagation of aggregates of misfolded α-synuclein in the brain following a specific pattern (Braak et al., 2006).
The relationship of this pattern with motor and cognitive symptoms is still equivocal. Therefore, we investigated longitudinally the spatio-temporal patterns of atrophy propagation in PD, their inter-individual variability and associations with clinical symptoms.
Magnetic resonance (MR) images of 37 PD patients and 27 controls were acquired at up to 15 time-points per subject, and over observation periods of up to 8.8 years (mean: 3.7 years). MR images were analyzed by Deformation-based Morphometry to measure region volumes and their longitudinal changes.
Differences of these regional volume data between patients and controls and their associations with clinical symptoms were calculated.
At baseline, group differences in the regional volumes were found mainly in areas of the sensory, motor and orbitofrontal cortices, areas in the frontal operculum, inferior frontal sulcus, hippocampus and entorhinal cortex, and in the substantia nigra, among others.
The longitudinal analysis yielded more widespread and more pronounced group differences, with significantly accelerated volume decreases in PD patients in the occipital and temporal lobes, the inferior parietal lobule, as well as in the insula, putamen and nucleus basalis Meynert.
The white matter was less affected than the gray matter. Worse clinical scores (MMSE, PDQ-39, UPDRS-III) were in particular associated with volume decreases of cortical areas, amygdala and basal forebrain nuclei, but not of the basal ganglia.
The observed longitudinal patterns of accelerated volume decrease in PD patients largely coincide with the pattern of α-synuclein pathology in PD stages 3–5 as proposed by Braak and colleagues. Thus, longitudinal DBM appears to depict already in-vivo the progression of neuropathological changes.






