Summary: Researchers say brain areas associated with neurodegenerative diseases tend to have less dense vascular networks which supply the brain with energy via the blood. The findings shed light on how the neurovascular system plays a role in the development of neurodegeneration.
Source: Penn State
Areas of the brain vulnerable to neurodegenerative disorders like Alzheimer’s disease tend to have less-dense vascular networks—which supply the brain with energy via blood—than other areas of the brain, according to Penn State researchers.
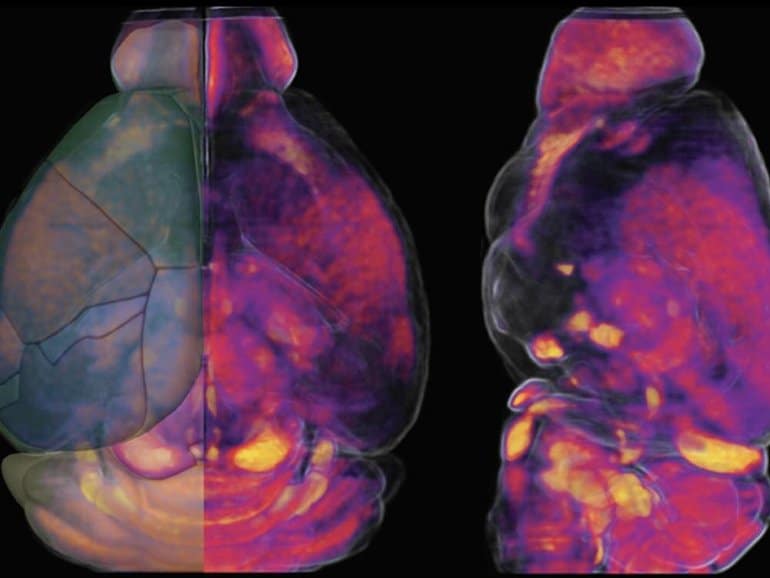
They developed new maps showing how vascular cells and structures differ across various mouse brain regions that may help them understand how vascular supply may relate to neurological diseases.
Brain health relies on efficient energy supply and waste removal via blood supplied by the body’s vascular system, which includes veins and arteries. Despite being only 2% of body weight, the brain consumes about 20% of body energy.
Dysfunctional vasculature has been implicated in many neurodegenerative disorders. Yet, scientists have limited knowledge on how the vascular network, which includes tiny microvessels, is organized across different brain areas, and the relationship between vasculature and key brain cell types, like neurons.
The research team, led in part by Yongsoo Kim, associate professor of neural and behavioral sciences at the Penn State College of Medicine, found that areas of the brain that process motor and sensory information have a high degree of the vasculature.
They also found that association areas of the brain, where different pieces of information are synthesized and processed, tend to have a lower degree of the vasculature.
“Loss or blocking of brain vessels, which occurs as people age, can further lower energy supply in regions that have a less robust vascular network at baseline,” Kim said.
“As a result, neurons in association areas may undergo accelerated cell death, which has been linked with cognitive decline.”
According to the researchers, studies to mitigate cognitive decline historically focused on targeting neurons to prevent cell loss, while less attention was given to understanding how the brain environment, namely energy supply, contributes to neurodegenerative processes.
They hypothesize that neuronal loss upon aging may be an outcome of dysfunctional energy infrastructure in the brain.
Kim said studying the brain’s vascular network is challenging due to the small size of the vessels and cells that make up the vasculature and its complex connections.
“It is like figuring out every single thread of a spider’s web and its connections in three dimensions,” Kim said.
To understand how the vascular network is organized and to understand its relationship with different neuronal cell types in a mammalian brain, the team used high resolution, three-dimensional mapping methods to examine the complete structure of the vasculature, pericytes (cells that control blood supply and vascular permeability) and key neuronal cell types in the adult mouse brain.
“Our approach is to generate a comprehensive map similar to a Google map to see detailed cellular structures in the context of the whole mouse brain.”
Using complex algorithms and serial two-photon tomography, a technique for imaging a cross-section of an organism at single-cell resolution, Kim and colleagues generated maps of the vasculature in a whole mouse brain. They also genetically labeled different brain cell types to compare the density and spatial location of vasculature across various regions.
They published their findings in Cell Reports on June 21.
Given their discovery that areas vulnerable to neurodegenerative diseases tend to have less dense vascular networks, Kim and his colleagues are comparing brain vascular network changes between normally aged mice as well as those that carry Alzheimer’s risk factors.
They hope to gather evidence to support their hypothesis that changes in brain vasculature can lead to neuronal dysfunction linked with cognitive decline. In the long term, their research might provide clues and therapeutic targets to prevent age-related brain damage.
“Understanding how energy balance and its malfunction can lead to brain disorders could help prevent age-related neurodegeneration,” Kim said.
“Our study provides an essential baseline to examine the relationship between vascular networks and neuronal cell types and how changes in these systems may contribute to different brain disorders.”
About this neurodegeneration research news
Author: Zach Sweger
Source: Penn State
Contact: Zach Sweger – Penn State
Image: The image is credited to Penn State College of Medicine
Original Research: Open access.
“Quantitative relationship between cerebrovascular network and neuronal cell types in mice” by Yuan-ting Wu et al. Cell Reports
Abstract
Quantitative relationship between cerebrovascular network and neuronal cell types in mice
Highlights
- Resource to examine cerebrovasculature, pericytes, and related neuronal subtypes
- Dense cerebrovasculature network in motor sensory circuits
- Cortical vasculature significantly correlated with parvalbumin and nNOS neurons
- Neurovascular architecture to meet regionally distinct brain energy regulation
Summary
The cerebrovasculature and its mural cells must meet brain regional energy demands, but how their spatial relationship with different neuronal cell types varies across the brain remains largely unknown.
Here we apply brain-wide mapping methods to comprehensively define the quantitative relationships between the cerebrovasculature, capillary pericytes, and glutamatergic and GABAergic neurons, including neuronal nitric oxide synthase-positive (nNOS+) neurons and their subtypes in adult mice.
Our results show high densities of vasculature with high fluid conductance and capillary pericytes in primary motor sensory cortices compared with association cortices that show significant positive and negative correlations with energy-demanding parvalbumin+ and vasomotor nNOS+ neurons, respectively.
Thalamo-striatal areas that are connected to primary motor sensory cortices also show high densities of vasculature and pericytes, suggesting dense energy support for motor sensory processing areas.
Our cellular-resolution resource offers opportunities to examine spatial relationships between the cerebrovascular network and neuronal cell composition in largely understudied subcortical areas.