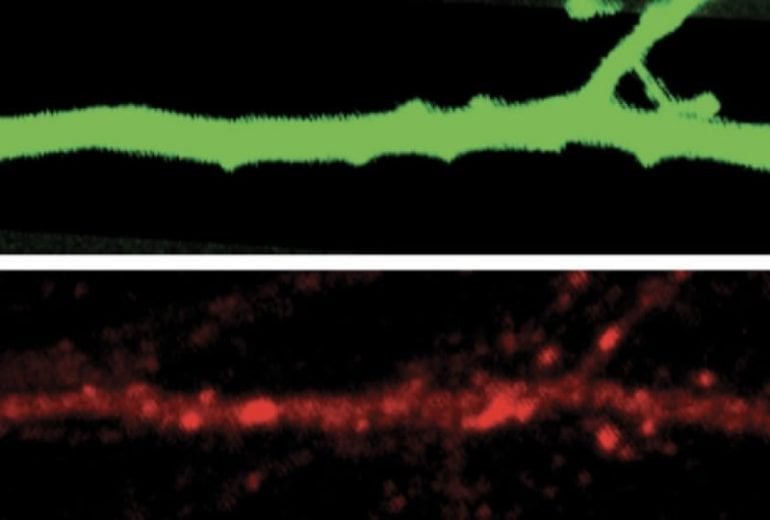
Summary: Findings support the idea that lithium, a drug more commonly associated with the treatment of bipolar disorder, may help treat those with SHANK3 related autism.
Source: Brandeis University
The mood-stabilizing drug lithium eases repetitive behaviors seen in mice missing SHANK3, an autism gene, according to a new study.
The findings suggest lithium merits further study as a treatment for some people with autism, even though the drug has troublesome side effects, including tremors and impaired memory.
“Lithium is, of course, a rather difficult, non-ideal treatment,” says lead investigator Gina Turrigiano, professor of vision science at Brandeis University in Waltham, Massachusetts. “It’s really hard to get people on a lithium regimen that they can tolerate well.” But understanding why lithium works may set the stage for better treatments, she says.
About 1 percent of people with autism have mutations in SHANK3. Deletion or mutation of the gene can also lead to Phelan-McDermid syndrome, which is characterized by intellectual disability, delayed speech and, often, autism.
Case studies of people with Phelan-McDermid syndrome also suggest that lithium eases behavior problems associated with the condition.
Previous work has shown that SHANK3 helps stabilize neuronal circuits by adjusting excitatory and inhibitory signaling like a thermostat. This process, called homeostatic plasticity, allows neurons to respond to changes in sensory input.
The new study suggests the gene controls this thermostat by regulating how frequently neurons fire and how they adjust the electrical currents flowing through them. SHANK3 loss or mutation prevents neurons from adapting to changes in sensory input.
“What we’ve shown is there’s this cellular mechanism, and loss of that mechanism is a direct result of a loss of SHANK3,” Turrigiano says.
Firepower
The team first recorded electrical activity in rat neurons in which SHANK3 expression was partially disrupted. They artificially blocked the neurons’ ability to fire. The mutant neurons’ rate of firing did not return to its original range afterward as it did in control cells, the team found.
The neurons also responded with less electrical activity than controls did when the researchers blocked and then restarted electrical currents. This suggests the mutant neurons had lost their ability to adjust the current flowing through them.
Excitatory neurons in the cortex called pyramidal cells are most strongly affected. Lithium restored the cells’ ability to adjust both their firing rates and how much current they carry.
To examine how neurons in mice lacking SHANK3 respond to changes in sensory input, the team implanted electrode arrays into the visual cortex of the mutant mice and controls and glued one eye shut in each mouse.
Over the following three or four days, the neurons in both groups of mice decreased how frequently they fired. But the decrease in the mutant mice was more gradual, suggesting that they take longer to adjust.
Neurons in the control mice returned to their typical firing rate two days after the rate had slowed to its lowest level, presumably because the mice’s brains had adapted to the loss of vision in one eye. But in the mutant mice, the neurons never returned to their original firing rates, indicating that without the gene, their brains could not adapt.
The mutant mice also groom themselves excessively, a behavior that is thought to align with obsessive or repetitive behaviors in people. To the researchers’ surprise, lithium stopped this behavior entirely. The work appeared in March in Neuron.
Treatment potential
The findings support the idea that lithium may help treat people with SHANK3 mutations, and perhaps those with other forms of autism as well, says Jean Martin Beaulieu, associate professor of psychiatry and neurosciences at the University of Toronto in Canada, who was not involved in the study.
However, he says, the study does not prove that the problems with homeostatic plasticity cause the repetitive behavior. Overgrooming is a complex behavior involving multiple brain areas, he says, whereas the study examined only the visual cortex.
It is also unclear whether lithium acts directly on SHANK3 or on some other target in the circuit, says Thomas Bourgeron, professor of genetics at the Institut Pasteur in Paris, France, who was not involved in the study.
Turrigiano says the loss of SHANK3 may be just the first step in a process that leads to neurons’ inability to adapt to sensory input. Identifying which part of that process lithium corrects may point to new targets for treatment, she says.
Turrigiano’s team is studying how SHANK3 helps balance excitatory and inhibitory activity of neurons, to better understand its role in maintaining homeostatic plasticity.
About this neuroscience research article
Source:
Brandeis University
Media Contacts:
Peter Hess – Brandeis University
Image Source:
The image is credited to Brandeis University.
Original Research: Closed access
“Autism-Associated Shank3 Is Essential for Homeostatic Compensation in Rodent V1”. by Vedakumar Tatavarty et al.
Neuron doi:10.1016/j.neuron.2020.02.033
Open access
“Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports”. by Sylvie Serret et al.
BMC Psychiatry doi:10.1186/s12888-015-0490-1
Abstract
Autism-Associated Shank3 Is Essential for Homeostatic Compensation in Rodent V1
Highlights
• Shank3 loss blocks synaptic and intrinsic homeostatic plasticity
• Li treatment rescues homeostatic plasticity after Shank3 loss
• Shank3 KO impairs homeostatic compensation to sensory deprivation
• Li rescues an overgrooming phenotype in Shank3 KO mice
Summary
Mutations in Shank3 are strongly associated with autism spectrum disorders and neural circuit changes in several brain areas, but the cellular mechanisms that underlie these defects are not understood. Homeostatic forms of plasticity allow central circuits to maintain stable function during experience-dependent development, leading us to ask whether loss of Shank3 might impair homeostatic plasticity and circuit-level compensation to perturbations. We found that Shank3 loss in vitro abolished synaptic scaling and intrinsic homeostatic plasticity, deficits that could be rescued by treatment with lithium. Further, Shank3 knockout severely compromised the in vivo ability of visual cortical circuits to recover from perturbations to sensory drive. Finally, lithium treatment ameliorated a repetitive self-grooming phenotype in Shank3 knockout mice. These findings demonstrate that Shank3 loss severely impairs the ability of central circuits to harness homeostatic mechanisms to compensate for perturbations in drive, which, in turn, may render them more vulnerable to such perturbations.
Abstract
Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports
Background
Phelan-Mc Dermid syndrome is a contiguous disorder resulting from 22q13.3 deletion implicating the SHANK3 gene. The typical phenotype includes neonatal hypotonia, moderate to severe intellectual disability, absent or delayed speech, minor dysmorphic features and autism or autistic-like behaviour. Recently, point mutations or micro-deletions of the SHANK3 gene have been identified, accompanied by a phenotype different from the initial clinically description in Phelan McDermid syndrome.
Case presentation
Here we present two case studies with similar psychiatric and genetic diagnosis as well as similar clinical history and evolution. The two patients were diagnosed with autism spectrum disorders in childhood and presented regression with catatonia features and behavioural disorders after a stressful event during adolescence. Interestingly, both patients presented mutation/microdeletion of the SHANK3 gene, inducing a premature stop codon in exon 21. Different pharmacological treatments (antipsychotics, benzodiazepines, mood stabilizer drugs, antidepressants, and methylphenidate) failed to improve clinical symptoms and lead to multiple adverse events. In contrast, lithium therapy reversed clinical regression, stabilized behavioural symptoms and allowed patients to recover their pre-catatonia level of functioning, without significant side effects.
Conclusion
These cases support the hypothesis of a specific SHANK3 phenotype. This phenotype might be linked to catatonia-like deterioration for which lithium use could be an efficient treatment. Therefore, these cases provide an important contribution to the field of autism research, clinical genetics and possible pharmacological answers.
Feel Free To Share This Autism News.