Summary: Findings from Rutgers researchers may help to develop new treatments for spinal cord injuries and neurodegenerative diseases.
Source: Rutgers University.
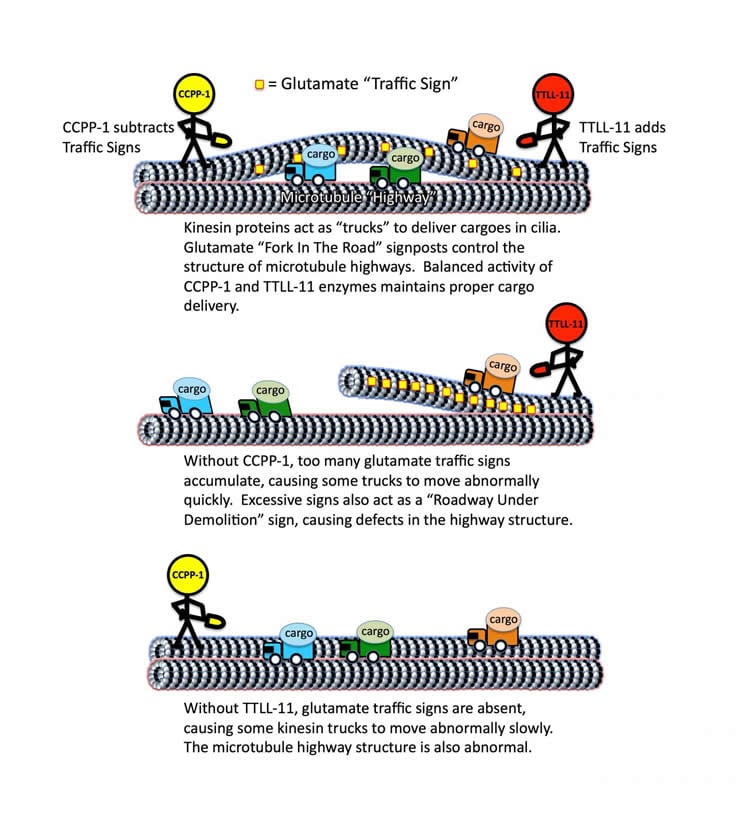
Inside cells, protein “motors” act like trucks on tiny cellular highways to deliver life-sustaining cargoes.
Now a team led by Rutgers University-New Brunswick researchers has discovered how cells deploy enzymes to place traffic control and “roadway under construction” signs along cellular highways.
“To stay alive and function, every cell in our body needs to transport cargoes to the place they’re needed inside the cell, in the right amount and at the right time,” said Robert O’Hagan, lead author of a new study and assistant research professor in the Human Genetics Institute of New Jersey and the Department of Genetics at Rutgers University-New Brunswick. “So there has to be a lot of organization in how transport inside the cell is regulated, and now we know a lot more about how that happens.”
The study, published online today in Current Biology, has implications for future therapies for spinal cord and nerve injuries and neurodegenerative diseases. Co-authors include Malan Silva, who earned a doctorate at Rutgers; Winnie Zhang, Sebastian Bellotti and Yasmin Ramadan, who received bachelor’s degrees at Rutgers; and Professor Maureen M. Barr, who heads the Barr Lab in the Human Genetics Institute of New Jersey and the Department of Genetics in the School of Arts and Sciences.
The highways inside cells are called microtubules, and proteins called kinesins and dyneins act like motors and are essentially the cargo trucks in cells, O’Hagan said. The motor proteins drive cargoes around microtubule highways, but a central question in cell biology is how intracellular transport and the highway systems are organized. Questions include how the motor proteins know where to go and how fast they need to be.
The Rutgers-led team studied C. elegans, a microscopic roundworm, and looked at microtubules in cilia – hair-like organelles that protrude from cells and perform sensory tasks. The scientists found that TTLL-11 is an enzyme that puts traffic signs composed of the amino acid glutamate on the microtubule highways to regulate the speed of the protein cargo trucks. CCPP-1 is an enzyme that takes down these glutamate traffic signs when there are too many of them, according to O’Hagan.
“Working together, they seem to regulate the speed of the motors that move cargoes on the microtubular highways,” he said.
The scientists also found that the glutamates can also act as a “roadway under construction” sign, changing the highways’ structure, he said.
Intriguingly, these enzymes are involved in degenerating cells that are crucial for vision, as well as neurons, O’Hagan said. The study suggests that future therapies targeting these enzymes might counter neurodegenerative diseases or nerve damage, including spinal cord injuries, he said.
“The picture that’s emerging from our and other labs’ research is that, for neurons to regenerate after injury or to survive in the brain, they need to be able to reorganize their microtubular highways and their cargo trucks in order to bring the right cargoes around to rebuild or maintain the cell,” he said.
“There’s a lot more to be discovered,” he added. “Our next step is to see how this works in the spinal cord in mammals, so we’ve started studies of rat spinal cord neurons.”
Funding: This research was funded by grants from the National Institutes of Health and the New Jersey Commission on Spinal Cord Research.
Source: Todd B. Bates – Rutgers University
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Robert O’Hagan/Rutgers University-New Brunswick.
Original Research: Abstract for “Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia” by Robert O’Hagan, Malan Silva, Ken C.Q. Nguyen, Winnie Zhang, Sebastian Bellotti, Yasmin H. Ramadan, David H. Hall,and Maureen M. Barr in Current Biololgy. Published online November 10 2017 doi:10.1016/j.cub.2017.09.066
[cbtabs][cbtab title=”MLA”]Rutgers University “How to Control Traffic on Cellular Highways.” NeuroscienceNews. NeuroscienceNews, 10 November 2017.
<https://neurosciencenews.com/enzyme-transportation-7915/>.[/cbtab][cbtab title=”APA”]Rutgers University (2017, November 10). How to Control Traffic on Cellular Highways. NeuroscienceNews. Retrieved November 10, 2017 from https://neurosciencenews.com/enzyme-transportation-7915/[/cbtab][cbtab title=”Chicago”]Rutgers University “How to Control Traffic on Cellular Highways.” https://neurosciencenews.com/enzyme-transportation-7915/ (accessed November 10, 2017).[/cbtab][/cbtabs]
Abstract
Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia
Highlights
•TTLL-11 and CCPP-1 fine-tune ciliary microtubule glutamylation
•Velocity of OSM-3/KIF17 and KLP-6/KIF28 motors is sensitive to glutamylation defects
•TTLL-11 and CCPP-1 are required for ciliary extracellular vesicle release
•TTLL-11 and CCPP-1 and microtubule glutamylation specialize ciliary ultrastructure
Summary
Ciliary microtubules (MTs) are extensively decorated with post-translational modifications (PTMs), such as glutamylation of tubulin tails. PTMs and tubulin isotype diversity act as a “tubulin code” that regulates cytoskeletal stability and the activity of MT-associated proteins such as kinesins. We previously showed that, in C. elegans cilia, the deglutamylase CCPP-1 affects ciliary ultrastructure, localization of the TRP channel PKD-2 and the kinesin-3 KLP-6, and velocity of the kinesin-2 OSM-3/KIF17, whereas a cell-specific α-tubulin isotype regulates ciliary ultrastructure, intraflagellar transport, and ciliary functions of extracellular vesicle (EV)-releasing neurons. Here we examine the role of PTMs and the tubulin code in the ciliary specialization of EV-releasing neurons using genetics, fluorescence microscopy, kymography, electron microscopy, and sensory behavioral assays. Although the C. elegans genome encodes five tubulin tyrosine ligase-like (TTLL) glutamylases, only ttll-11 specifically regulates PKD-2 localization in EV-releasing neurons. In EV-releasing cephalic male (CEM) cilia, TTLL-11 and the deglutamylase CCPP-1 regulate remodeling of 9+0 MT doublets into 18 singlet MTs. Balanced TTLL-11 and CCPP-1 activity fine-tunes glutamylation to control the velocity of the kinesin-2 OSM-3/KIF17 and kinesin-3 KLP-6 without affecting the intraflagellar transport (IFT) kinesin-II. TTLL-11 is transported by ciliary motors. TTLL-11 and CCPP-1 are also required for the ciliary function of releasing bioactive EVs, and TTLL-11 is itself a novel EV cargo. Therefore, MT glutamylation, as part of the tubulin code, controls ciliary specialization, ciliary motor-based transport, and ciliary EV release in a living animal. We suggest that cell-specific control of MT glutamylation may be a conserved mechanism to specialize the form and function of cilia.
“Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia” by Robert O’Hagan, Malan Silva, Ken C.Q. Nguyen, Winnie Zhang, Sebastian Bellotti, Yasmin H. Ramadan, David H. Hall,and Maureen M. Barr in Current Biololgy. Published online November 10 2017 doi:10.1016/j.cub.2017.09.066






