Summary: According to researchers, a genetic variant changes the expression of the SNAP25 protein, impacting information processing between brain areas associated with regulating emotions. Researchers report this may increase the risk of developing schizophrenia and bipolar disorder.
Source: SfN.
A genetic variant associated with multiple psychiatric disorders drives changes in a brain network that may increase an individual’s risk of developing bipolar disorder and schizophrenia, finds a study published in JNeurosci.
Stéphane Jamain and colleagues used genetic analysis and neuroimaging in samples of adults with schizophrenia, early-onset bipolar disorder and healthy controls — in addition to postmortem analysis of brain tissue from schizophrenia patients — to demonstrate that a variant of a gene involved in neurotransmission is associated with both disorders.
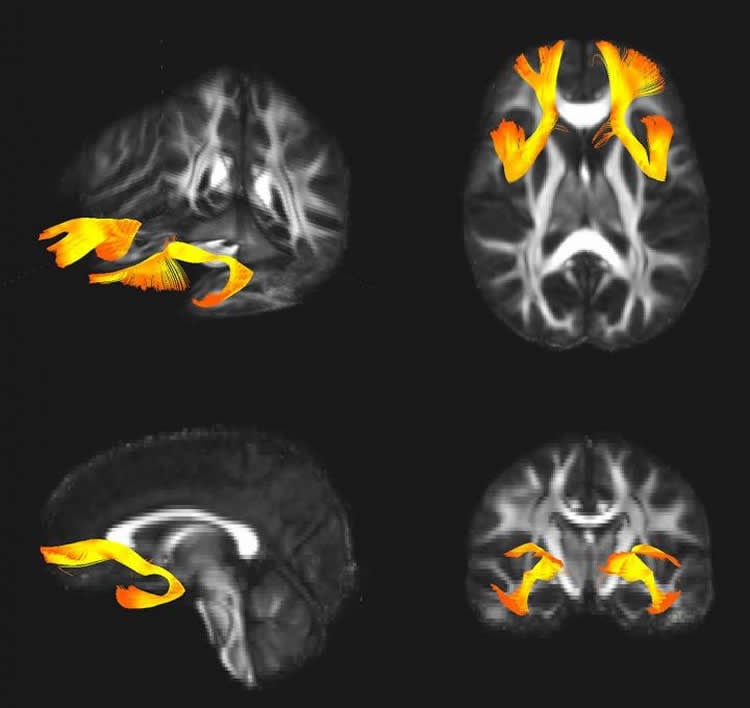
They found that this genetic variation changes the expression of the SNAP25 protein in the brain, which may impact information processing between brain regions involved in regulating emotions. Consistent with this idea, the variant was associated with larger amygdala volume and altered prefrontal-limbic connectivity.
The authors replicated these findings in independent samples, minimizing the potential for false positives and increasing confidence in their results.
The research confirms a shared genetic component of these disorders and points to a potentially new condition that may arise in patients with varying diagnoses in which this gene is implicated, such as attention-deficit/hyperactivity disorder.
Funding: Funding provided by Agence Nationale pour la Recherche, Institut National pour la Santé et la Recherche Médicale, Réseau Thématique de Recherche et de Soins en Santé Mentale, Institut Thématique Multi Organismes Neurosciences.
Source: David Barnstone – SfN
Image Source: NeuroscienceNews.com image is credited to Stéphane Jamain.
Original Research: Abstract for “A multi-level functional study of a SNAP25 at-risk variant for bipolar disorder and schizophrenia” by Josselin Houenou, Jennifer Boisgontier, Annabelle Henrion, Marc-Antoine d’Albis, Anne Dumaine, Julia Linke, Michèle Wessa, Claire Daban, Nora Hamdani, Marine Delavest, Pierre-Michel Llorca, Christophe Lançon, Franck Schürhoff, Andrei Szöke, Philippe Le Corvoisier, Caroline Barau, Cyril Poupon, Bruno Etain, Marion Leboyer and Stéphane Jamain in Journal of Neuroscience. Published online October 2 2017 doi:10.1523/JNEUROSCI.1040-17.2017
[cbtabs][cbtab title=”MLA”]SfN “Shared Genetics in Schizophrenia and Bipolar Disorder.” NeuroscienceNews. NeuroscienceNews, 2 October 2017.
<https://neurosciencenews.com/bipolar-schizophrenia-genetics-7637/>.[/cbtab][cbtab title=”APA”]SfN (2017, October 2). Shared Genetics in Schizophrenia and Bipolar Disorder. NeuroscienceNews. Retrieved October 2, 2017 from https://neurosciencenews.com/bipolar-schizophrenia-genetics-7637/[/cbtab][cbtab title=”Chicago”]SfN “Shared Genetics in Schizophrenia and Bipolar Disorder.” https://neurosciencenews.com/bipolar-schizophrenia-genetics-7637/ (accessed October 2, 2017).[/cbtab][/cbtabs]
Abstract
A multi-level functional study of a SNAP25 at-risk variant for bipolar disorder and schizophrenia
The synaptosomal associated protein SNAP25 is a key player in synaptic vesicle docking and fusion and has been associated with multiple psychiatric conditions, including schizophrenia, bipolar disorder and attention-deficit/hyperactivity disorder. We recently identified a promoter variant in SNAP25, rs6039769, associated with early-onset bipolar disorder and a higher gene expression level in human prefrontal cortex. In the current study, we showed that this variant was associated both in males and females with schizophrenia in two independent cohorts. We then combined in vitro and in vivo approaches in humans to understand the functional impact of the at-risk allele. Thus, we showed in vitro that the rs6039769 C allele was sufficient to increase the SNAP25 transcription level. In a postmortem expression analysis of 33 individuals affected with schizophrenia and 30 unaffected controls, we showed that the SNAP25b:SNAP25a ratio was increased in schizophrenic patients carrying the rs6039769 at-risk allele. Last, using genetics imaging in a cohort of 71 subjects, we showed that male risk carriers had an increased amygdala-ventromedial prefrontal cortex functional connectivity and a larger amygdala than non-risk carriers. The latter association has been replicated in an independent cohort of 121 independent subjects. Altogether, results from these multi-level functional studies are bringing strong evidence for the functional consequences of this allelic variation of SNAP25 on modulating the development and plasticity of the prefrontal-limbic network, which therefore may increase the vulnerability to both early onset bipolar disorder and schizophrenia.
SIGNIFICANCE STATEMENT
Functional characterization of disease-associated variants is a key challenge in understanding neuropsychiatric disorders and will open avenue in the development of personalized treatments. Recent studies have cumulated evidence that the SNARE complex and more specifically the SNAP25 protein may be involved in psychiatric disorders. Here, our multi-level functional studies are bringing strong evidence for the functional consequences of an allelic variation of SNAP25 on modulating the development and plasticity of the prefrontal-limbic network. These results demonstrate a common genetically-driven functional alteration of a synaptic mechanism both in schizophrenia and early-onset bipolar disorder, and confirm the shared genetic vulnerability between these two disorders.
“A multi-level functional study of a SNAP25 at-risk variant for bipolar disorder and schizophrenia” by Josselin Houenou, Jennifer Boisgontier, Annabelle Henrion, Marc-Antoine d’Albis, Anne Dumaine, Julia Linke, Michèle Wessa, Claire Daban, Nora Hamdani, Marine Delavest, Pierre-Michel Llorca, Christophe Lançon, Franck Schürhoff, Andrei Szöke, Philippe Le Corvoisier, Caroline Barau, Cyril Poupon, Bruno Etain, Marion Leboyer and Stéphane Jamain in Journal of Neuroscience. Published online October 2 2017 doi:10.1523/JNEUROSCI.1040-17.2017






