Summary: Newly designed antibodies that bind to small clumps of amyloid-beta may keep the progression of Alzheimer’s disease in check.
Source: Uppsala University
Researchers at Uppsala University have designed new antibodies that might provide more effective treatment methods for Alzheimer’s disease. By designing antibodies that bind even to the smaller aggregates, or clumps, of the amyloid-beta protein, it may be possible to check the progress of the disease.
Developing effective treatment methods for Alzheimer’s disease has proved difficult. The most effective, which have just been approved, only provide marginal effects. There are several major reasons why they are not effective, one of which is that the antibodies used do not bind to all the types of toxic clumps that cause Alzheimer’s disease.
In Alzheimer’s disease, the amyloid-beta protein begins to form clumps. This process is called aggregation, and the clumps created are called aggregates. The research group has previously shown that treatment with the peptide somatostatin causes the body to begin breaking down building blocks of the aggregate. In the new study, the researchers use an antibody that can bind to the toxic aggregates to stop them from harming cells.
The problem with the treatment methods currently tested in patient studies is that the antibodies bind much more strongly to large clumps and hardly at all to small clumps. The small clumps are just as toxic as the large ones and many think that they are actually even more dangerous since they can move more.
The purpose of the current study was to develop an antibody format that can bind to both large and small clumps of amyloid-beta. Antibodies use the avidity effect to bind strongly to their targets. This requires the binding of both arms of the antibody to the same target at the same time (see figure).
The distance between the arms of the antibody is crucial for how small an aggregate the antibody can bind to strongly. If the aggregate is smaller than the distance between the arms, they do not bind to the aggregate strongly. If it is larger, they bind to the aggregate very strongly. In the new article, the researchers have developed a new antibody format with shorter distances between the arms so that they bind to smaller aggregates. The new format also has more binding sites to make the binding extra strong.
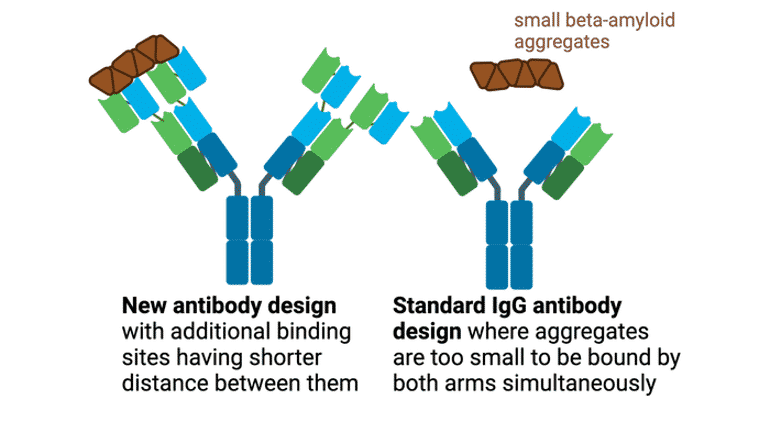
“Thanks to the avidity effect, the new antibody format is at least 40 times stronger in binding to the clumps. The new type of antibody can also bind to small aggregates with avidity, which we have not previously seen any other antibody do. That is fantastic,” says Greta Hultqvist, Senior lecturer and Associate Professor in Protein drug design at Uppsala University who led the study.
The effects of the antibodies were also tested in a cell culture experiment, which showed that the new antibody format could save cells from death caused by amyloid-beta aggregates. Although no pre-clinical experiments were included, the team thinks their results suggest that the new antibody design could be more effective than those trialled so far.
“The focus of the study was targeting the amyloid-beta protein in Alzheimer’s disease, but the new antibody design can be general and applicable to other disease-causing clumps. From a long-term perspective, we hope that the new format can open up new avenues for the generation of future treatments, not only in Alzheimer’s disease, but also other diseases where proteins start to form aggregates, like Parkinson’s disease,” says Fadi Rofo, doctoral student and first author of the study.
About this Alzheimer’s disease research news
Author: Elin Backstrom
Source: Uppsala University
Contact: Elin Backstrom – Uppsala University
Image: The image is in the public domain
Original Research: Open access.
“Novel multivalent design of a monoclonal antibody improves binding strength to soluble aggregates of amyloid beta” by Greta Hultqvist et al. Translational Neurodegeneration
Abstract
Novel multivalent design of a monoclonal antibody improves binding strength to soluble aggregates of amyloid beta
Background
Amyloid-β (Aβ) immunotherapy is a promising therapeutic strategy in the fight against Alzheimer’s disease (AD). A number of monoclonal antibodies have entered clinical trials for AD. Some of them have failed due to the lack of efficacy or side-effects, two antibodies are currently in phase 3, and one has been approved by FDA. The soluble intermediate aggregated species of Aβ, termed oligomers and protofibrils, are believed to be key pathogenic forms, responsible for synaptic and neuronal degeneration in AD. Therefore, antibodies that can strongly and selectively bind to these soluble intermediate aggregates are of great diagnostic and therapeutic interest.
Methods
We designed and recombinantly produced a hexavalent antibody based on mAb158, an Aβ protofibril-selective antibody. The humanized version of mAb158, lecanemab (BAN2401), is currently in phase 3 clinical trials for the treatment of AD. The new designs involved recombinantly fusing single-chain fragment variables to the N-terminal ends of mAb158 antibody. Real-time interaction analysis with LigandTracer and surface plasmon resonance were used to evaluate the kinetic binding properties of the generated antibodies to Aβ protofibrils. Different ELISA setups were applied to demonstrate the binding strength of the hexavalent antibody to Aβ aggregates of different sizes. Finally, the ability of the antibodies to protect cells from Aβ-induced effects was evaluated by MTT assay.
Results
Using real-time interaction analysis with LigandTracer, the hexavalent design promoted a 40-times enhanced binding with avidity to protofibrils, and most of the added binding strength was attributed to the reduced rate of dissociation. Furthermore, ELISA experiments demonstrated that the hexavalent design also had strong binding to small oligomers, while retaining weak and intermediate binding to monomers and insoluble fibrils. The hexavalent antibody also reduced cell death induced by a mixture of soluble Aβ aggregates.
Conclusion
We provide a new antibody design with increased valency to promote binding avidity to an enhanced range of sizes of Aβ aggregates. This approach should be general and work for any aggregated protein or repetitive target.