Summary: PTPδ, a synaptic adhesion molecule, regulates synaptic development and sleep behavior in mouse models.
Source: Institute for Basic Science
Plenty of theories, but nobody knows exactly why we sleep. One thing we know for sure: sleepless nights will not help you make it through the day. There have been many studies exploring how sleep works in the brain and what its purposes are. However, how the synapse – the fundamental building block of the brain contributing to the formation of neuronal circuits – works in relation to sleep has been poorly understood. A research team led by Director KIM Eunjoon of the Center for Synaptic Brain Dysfunctions within the Institute for Basic Science (IBS, South Korea), has reported in vivo findings that a presynaptic cell adhesion molecule named PTPδ is crucial for the development of synapses in the developing brain. Thus, the genetic deletion of PTPδ leads to disruptions in structural, functional, and biochemical compositions in the brain of the afflicted mice, resulting in changes in innate behaviors, such as hyper-locomotor activity, increased anxiety, and decreased sleep.
The human brain contains billions of neurons which communicate with each other via synapses. The signals one neuron sends to another via the release of neurotransmitters at presynaptic sites are sensed by neurotransmitter receptors located at postsynaptic sites of other neurons. In turn, networks of neurons determine the expression of all behaviors and bodily functions controlled by the brain. At the core of all these comprehensive networks for brain functions are the synaptic cell adhesion molecules (CAMs). CAMs are essential for regulating the development of synapses by bridging the pre- and postsynaptic sides. To ensure normal development of neuronal circuits and brain functions, it is critical to make appropriate paring of pre- and postsynaptic CAMs, among many different kinds, during the development of young neurons.
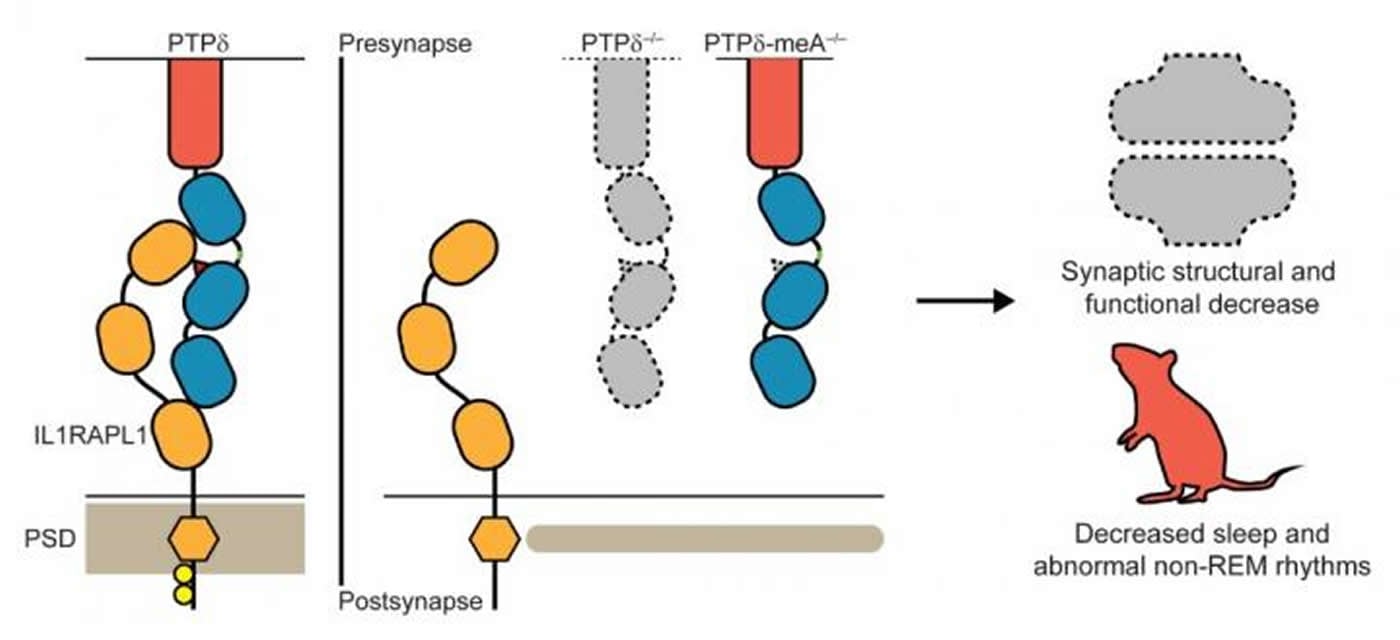
The research team focused on finding specific roles of PTPδ, as it was only suspected of playing a key role in synaptic formation. They developed a fluorescent molecule-tagged version of PTPδ, whose expression in the brain allowed precise and clear-cut visualization of the natural location of PTPδ across the brain and down to the position within the synapse with nanometer scale precision. They also adopted conditional deletions of PTPδ to pinpoint the key joint component in synaptic formation. Crucially, the team has demonstrated that disruptions of presynaptic PTPδ interaction with its postsynaptic binding partner IL1RAPL1 cause all key changes in innate behaviors as shown in Figures 2 and 3.
It has been previously shown that the trans-synaptic interaction (the interaction that spans across the synapse from one neuron to the next) depends on a six amino acid-long short peptide sequence (known as meA) within the PTPδ protein. The research team used this knowledge to specifically block the PTPδ-IL1RAPL1 interaction. When the key meA sequence is deleted, leaving the rest of PTPδ functional and intact, IL1RAPL1 is unable bind to PTPδ and therefore unable to stay on the postsynaptic side of a synapse. This leads to a significant decrease in the number of synapses across the brain, disrupting the intricate neuronal circuits that form and determine behavior output such as sleep. They also selectively deleted PTPδ on excitatory neurons and confirmed the hypothesis that the synapse-to-behavior disruptions were indeed localized to excitatory neurons.
The research team also showed that when IL1RAPL1 is unable to bind to PTPδ across the synapse, the chemical composition of IL1RAPL1 itself changes, with near-complete block of a process called tyrosine phosphorylation in its amino acid sequence. This change in the innate property of IL1RAPL1 is accompanied by exclusion of IL1RAPL1 from the synapse. Because IL1RAPL1 is crucial for the maturation of the synapse, this exclusion leads to structural and functional decreases of the synapse. Disruption of the brain at such a fundamental level leads to the increase in anxiety and decrease in sleep seen in the PTPδ-mutant mice.
There have been several studies that linked synaptic CAMs with abnormal mouse behaviors. Notably, this study offers the first comprehensive overview of how CAMs are linked with sleep, a fundamental brain function. In addition, no previous studies have associated a specific pair of pre- and postsynaptic CAMs with sleep or sleep or sleep-related behaviors such as hyperactivity and anxiety. As mutations in the gene coding for PTPδ are associated with numerous psychiatric disorders, such as schizophrenia, attention deficit hyperactivity disorder (ADHD), and restless leg syndrome (a type of sleep disorder) with each of these disorders affecting 1% to 5% of the general population worldwide, this study shines light on how changes in PTPδ may lead to symptoms reminiscent of the aforementioned disorders.
The development of the brain during the embryonic and early postnatal stages are crucial for the proper expression of behavior and brain-related functions. This study shows that even the singular disruption of the PTPδ-IL1RAPL1 interaction, among many possible pairs of pre- and postsynaptic CAMs, has consequences that last the entire lifetime of the organism, such as a permanent reduction of sleep. Given that sleep is essential to survival, the disruption of a single binding pair at the synapse can have such far-reaching consequences speaks of the importance of this discovery, which will further our understanding of how the basic building blocks of the brain influences complex behaviors such as sleep.
About this neuroscience research article
Source:
Institute for Basic Science
Media Contacts:
Eunjoon Kim – Institute for Basic Science
Image Source:
The image is credited to Institute for Basic Science.
Original Research: Open access
“Splice‐dependent trans‐synaptic PTPδ–IL1RAPL1 interaction regulates synapse formation and non‐REM sleep”. by Eunjoon Kim et al.
EMBO Journal doi:10.15252/embj.2019104150
Abstract
Splice‐dependent trans‐synaptic PTPδ–IL1RAPL1 interaction regulates synapse formation and non‐REM sleep
Alternative splicing regulates trans‐synaptic adhesions and synapse development, but supporting in vivo evidence is limited. PTPδ, a receptor tyrosine phosphatase adhering to multiple synaptic adhesion molecules, is associated with various neuropsychiatric disorders; however, its in vivo functions remain unclear. Here, we show that PTPδ is mainly present at excitatory presynaptic sites by endogenous PTPδ tagging. Global PTPδ deletion in mice leads to input‐specific decreases in excitatory synapse development and strength. This involves tyrosine dephosphorylation and synaptic loss of IL1RAPL1, a postsynaptic partner of PTPδ requiring the PTPδ‐meA splice insert for binding. Importantly, PTPδ‐mutant mice lacking the PTPδ‐meA insert, and thus lacking the PTPδ interaction with IL1RAPL1 but not other postsynaptic partners, recapitulate biochemical and synaptic phenotypes of global PTPδ‐mutant mice. Behaviorally, both global and meA‐specific PTPδ‐mutant mice display abnormal sleep behavior and non‐REM rhythms. Therefore, alternative splicing in PTPδ regulates excitatory synapse development and sleep by modulating a specific trans‐synaptic adhesion.
Feel Free To Share This Neuroscience News.