Summary: A new study sheds light on how the brain performs the go/stop balance.
Source: Hong Kong University of Science and Technology.
As with all things in life, healthy brain function depends on a balance of forces. We think of our brains as active – moving a leg and saying a word are all “active” events. But it is just as important for our brains to be able to stop these actions -to stop our leg, and to stop our speech. But how do our brains actually perform the go/stop function?
In a recent study, an interdisciplinary team of scientists from The Hong Kong University of Science and Technology (HKUST) and The Chinese University of Hong Kong (CUHK) discovered that two large protein kinases, ATM and ATR, cooperate to help establish the go/stop balance.
The findings were published in the Journal Proceedings of the National Academy of Sciences on Jan 9, 2018.
“Our discovery offers a fresh perspective on how our brain balances excitation and inhibition – basically “go” and “stop” of behavior,” said Aifang Cheng, first author of the paper. “We show that ATM and ATR regulate each other’s levels in the brain. When ATM levels drop, ATR levels increase and the reverse. Just as important, regular brain activity also changes the levels of the two proteins. This means that neuronal activity and the two kinases are in a dynamic ‘conversation’ that helps to keep the appropriate balance between excitation and inhibition (known as the E/I balance) by adjusting the levels of ATM and ATR.” Just as important, the team found that the two proteins divide up responsibilities for the ‘go’ and ‘stop’ functions. ATM helps regulate only excitatory events while ATR helps regulate only the inhibitory ones; this is achieved by controlling the movement of tiny synaptic vesicles in the neuronal synapse – the gap between two neurons that regulates information flow in the brain.
Utilizing super-resolution microscopy offered by the Super-Resolution Imaging Center (SRIC) at HKUST, the researchers were able to view the cellular location of the two kinases at ultra-high magnification. The custom designed super-resolution microscope with active stage locking provided the stability needed to obtain high-resolution images. The groups had previously worked together to show that ATM was found on synaptic vesicles, but no one had ever looked for ATR. Combining their efforts for a second time, the team was able to show that ATR was also on synaptic vesicles (identified with a protein called VAMP2).
“One of the challenges we faced was that even at high magnification, all vesicles look pretty much alike,” said Du Shengwang, physics professor and Associate Director of SRIC. “To provide differentiation, we developed a three-color version of our super-resolution system, which allowed the team to prove that ATM and ATR were never found on the same VAMP2-containing synaptic vesicle.”
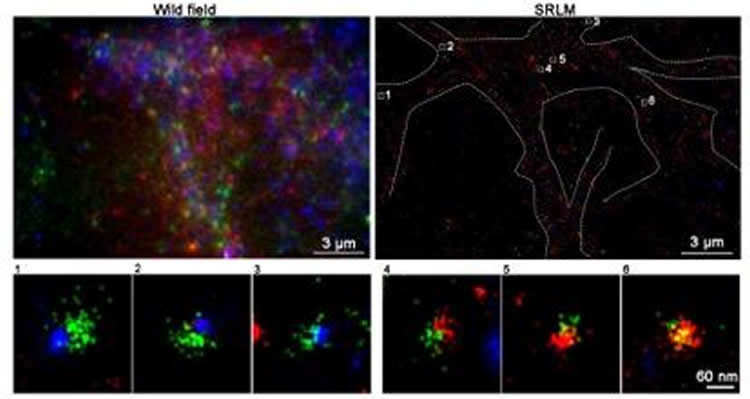
The HKUST team then sent their findings to their collaborators at CUHK, where they produced crisp, high-resolution images of normal synapses and synapses from neurons that had no ATM protein. This allowed Cheng to measure the size of the synaptic vesicles, and she discovered that the vesicles without ATM were bigger than normal, a hint that there was a problem with the composition of the synaptic membrane.
“The new findings are in the realm of basic research, but they have important implications for human disease,” said Karl Herrup, Chair Professor and Head of the Division of Life Science at HKUST, Director of the SRIC and the senior author of the manuscript. “Epilepsy, for example, is a condition where one of the problems is that inhibition fails. As our findings would predict, humans with too little ATR have a problem with epilepsy, while people with ATM deficiency by contrast are ataxic – a reduced ability to make finely controlled movements and keep the proper E/I ratio. This means that there is a yin-yang relationship between ATM and ATR. And really, this is only the beginning. We believe that our work has potential relevance to a much broader range of neurologic conditions.”
Funding: The work was supported by Research Grants Council, Hong Kong Special Administrative Region, Hong Kong University of Science and Technology, the National Key Basic Research Program of China, National Institutes of Health.
Source: Etta Lai – Hong Kong University of Science and Technology
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Division of Life Science, HKUST.
Original Research: Abstract in PNAS.
doi:10.1073/pnas.1716892115
[cbtabs][cbtab title=”MLA”]Hong Kong University of Science and Technology “New Insights Into How the Brain Keeps Itself Balanced.” NeuroscienceNews. NeuroscienceNews, 23 January 2018.
<https://neurosciencenews.com/balance-brain-8361/>.[/cbtab][cbtab title=”APA”]Hong Kong University of Science and Technology (2018, January 23). New Insights Into How the Brain Keeps Itself Balanced. NeuroscienceNews. Retrieved January 23, 2018 from https://neurosciencenews.com/balance-brain-8361/[/cbtab][cbtab title=”Chicago”]Hong Kong University of Science and Technology “New Insights Into How the Brain Keeps Itself Balanced.” https://neurosciencenews.com/balance-brain-8361/ (accessed January 23, 2018).[/cbtab][/cbtabs]
Abstract
ATM and ATR play complementary roles in the behavior of excitatory and inhibitory vesicle populations
ATM (ataxia-telangiectasia mutated) and ATR (ATM and Rad3-related) are large PI3 kinases whose human mutations result in complex syndromes that include a compromised DNA damage response (DDR) and prominent nervous system phenotypes. Both proteins are nuclear-localized in keeping with their DDR functions, yet both are also found in cytoplasm, including on neuronal synaptic vesicles. In ATM- or ATR-deficient neurons, spontaneous vesicle release is reduced, but a drop in ATM or ATR level also slows FM4-64 dye uptake. In keeping with this, both proteins bind to AP-2 complex components as well as to clathrin, suggesting roles in endocytosis and vesicle recycling. The two proteins play complementary roles in the DDR; ATM is engaged in the repair of double-strand breaks, while ATR deals mainly with single-strand damage. Unexpectedly, this complementarity extends to these proteins’ synaptic function as well. Superresolution microscopy and coimmunoprecipitation reveal that ATM associates exclusively with excitatory (VGLUT1+) vesicles, while ATR associates only with inhibitory (VGAT+) vesicles. The levels of ATM and ATR respond to each other; when ATM is deficient, ATR levels rise, and vice versa. Finally, blocking NMDA, but not GABA, receptors causes ATM levels to rise while ATR levels respond to GABA, but not NMDA, receptor blockade. Taken together, our data suggest that ATM and ATR are part of the cellular “infrastructure” that maintains the excitatory/inhibitory balance of the nervous system. This idea has important implications for the human diseases resulting from their genetic deficiency.






