Summary: Your sleep history during middle age may predict Alzheimer’s pathology later in life. A decrease in sleep quality between 50 and 70 years of age is associated with higher levels of tau and amyloid beta in the brain. Changes in brain activity and quality of sleep could be a biomarker for Alzheimer’s disease.
Source: SfN
Sleep patterns can predict the accumulation of Alzheimer’s pathology proteins later in life, according to a new study of older men and women published in Journal of Neuroscience. These findings could lead to new sleep-based early diagnosis and prevention measures in the treatment of Alzheimer’s disease.
Alzheimer’s disease is associated with disrupted sleep and the accumulation of tau and proteins in the brain, which can emerge long before characteristic memory impairments appear. Two types of hippocampal sleep waves, slow oscillations and sleep spindles, are synced in young individuals but have been shown to become uncoordinated in old age.
Matthew Walker, Joseph Winer, and colleagues at the University of California, Berkeley found a decrease in slow oscillations/sleep spindle synchronization was associated with higher tau, while reduced slow-wave-activity amplitude was associated with higher β-amyloid levels.
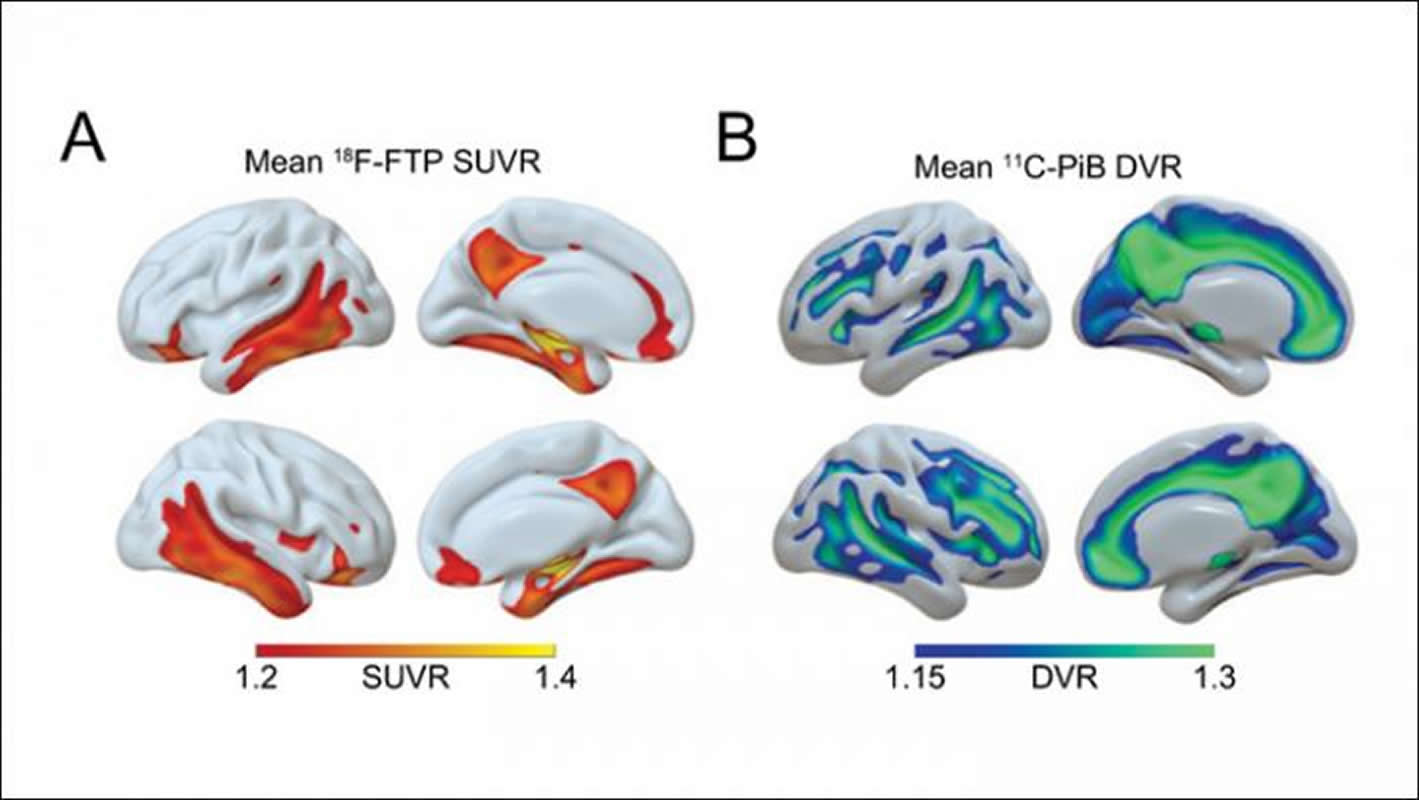
The researchers also found that a decrease in sleep quantity throughout aging, from the 50s through 70s, was associated with higher levels of β-amyloid and tau later in life. This means that changes in brain activity during sleep and sleep quantity during these time frames could serve as a warning sign for Alzheimer’s disease, allowing for early preventive care.
Source:
SfN
Media Contacts:
Calli McMurray – SfN
Image Source:
The image is credited to Winer et al., JNeurosci 2019.
Original Research: Closed access
“Sleep as a potential biomarker of tau and β-amyloid burden in the human brain”. Joseph R. Winer, Bryce A. Mander, Randolph F. Helfrich, Anne Maass, Theresa M. Harrison, Suzanne L. Baker, Robert T. Knight, William J. Jagust and Matthew P. Walker.
Journal of Neuroscience. doi:10.1523/JNEUROSCI.0503-19.2019
Abstract
Sleep as a potential biomarker of tau and β-amyloid burden in the human brain
Recent proposals suggest that sleep may be a factor associated with accumulation of two core pathological features of Alzheimer’s disease (AD): tau and β-amyloid (Aβ). Here we combined positron emission tomography measures of Aβ and tau, electroencephalogram sleep recordings, and retrospective sleep evaluations to investigate the potential utility of sleep measures in predicting in vivo AD pathology in male and female older adults. Regression analyses revealed that the severity of impaired slow oscillation-sleep spindle coupling predicted greater medial temporal lobe tau burden. Aβ burden was not associated with coupling impairment, but instead predicted the diminished amplitude of <1Hz slow-wave-activity—results that were statistically dissociable from each other. Additionally, comparisons of AD pathology and retrospective, self-reported changes in sleep duration demonstrated that changes in sleep across the lifespan can predict late-life Aβ and tau burden. Thus, quantitative and qualitative features of human sleep represent potential non-invasive, cost-effective and scalable biomarkers (current and future-forecasting) of AD pathology, and carry both therapeutic and public-health implications.
SIGNIFICANCE STATEMENT
Several studies have linked sleep disruption to the progression of Alzheimer’s disease (AD). Tau and β-amyloid (Aβ), the primary pathological features of AD, are associated with both objective and subjective changes in sleep. However it remains unknown whether late life tau and Aβ burden are associated with distinct impairments in sleep physiology or changes in sleep across the lifespan. Using polysomnography, retrospective questionnaires, and tau- and Aβ-specific positron emission tomography, the present study reveals human sleep signatures which dissociably predict levels of brain tau and Aβ in older adults. These results suggest that a night of polysomnography may aid in evaluating tau and Aβ burden, and that treating sleep deficiencies within decade-specific time windows may serve in delaying AD progression.






