Summary: Researchers identify a biological pathway for depression in high risk teens who come from socioeconomically disadvantaged families.
Source: Duke.
New study of high-risk teens reveals a biological pathway for depression.
A long line of research links poverty and depression. Now, a study by Duke University scientists shows how biology might underlie the depression experienced by high-risk adolescents whose families are socio-economically disadvantaged.
The study, published May 24, 2016 in the journal Molecular Psychiatry, combined genetics, brain imaging and behavioral data gathered as adolescents were followed for more than three years as part of a larger study.
The results are part of a growing body of work that may lead to biological predictors that could guide individualized depression-prevention strategies.
Adolescents growing up in households with lower socioeconomic status were shown to accumulate greater quantities of a chemical tag on a depression-linked gene over the course of two years. These “epigenetic” tags work by altering the activity of genes. The more chemical tags an individual had near a gene called SLC6A4, the more responsive was their amygdala — a brain area that coordinates the body’s reactions to threat — to photographs of fearful faces as they underwent functional MRI brain scans. Participants with a more active amygdala were more likely to later report symptoms of depression.
“This is some of the first research to demonstrating that low socioeconomic status can lead to changes in the way genes are expressed, and it maps this out through brain development to the future experience of depression symptoms,” said the study’s first author Johnna Swartz, a Duke postdoctoral researcher in the lab of Ahmad Hariri, a Duke professor of psychology and neuroscience.
Adolescence is rarely an easy time for anyone. But growing up in a family with low socioeconomic status or SES — a metric that incorporates parents’ income and education levels — can add chronic stressors such as family discord and chaos, and environmental risks such as poor nutrition and smoking.
“These small daily hassles of scraping by are evident in changes that build up and affect children’s development,” Swartz said.
The study included 132 non-Hispanic Caucasian adolescents in the Teen Alcohol Outcomes Study (TAOS) who were between 11 and 15 years old at the outset of the study and came from households that ranged from low to high SES. About half of the participants had a family history of depression.
“The biggest risk factor we have currently for depression is a family history of the disorder,” said study co-author Douglas Williamson, principal investigator of TAOS and professor of psychiatry and behavioral sciences at Duke. “Our new work reveals one of the mechanisms by which such familial risk may be manifested or expressed in a particular group of vulnerable individuals during adolescence.”
The group’s previous work, published last year in the journal Neuron, had shown that fMRI scan activity of the amygdala could signal who is more likely to experience depression and anxiety in response to stress several years later. That study included healthy college-aged participants of Hariri’s ongoing Duke Neurogenetics Study (DNS), which aims to link genes, brain activity, and other biological markers to a risk for mental illness.
This study asked whether higher activity in the same brain area could predict depression in the younger, at-risk TAOS participants. Indeed, about one year later, these individuals (now between 14 and 19 years of age) were more likely to report symptoms of depression, especially if they had a family history of the disorder.
Swartz said the new study examined a range of socioeconomic status and did not focus specifically on families affected by extreme poverty or neglect. She said the findings suggest that even modestly lower socioeconomic status is associated with biological differences that elevate adolescents’ risk for depression.
Most of the team’s work so far has focused on epigenetic chemical tags near the SLC6A4 gene because it helps control the brain’s levels of serotonin, a neurochemical involved in clinical depression and other mood disorders. The more marks present just upstream of this gene, the less likely it is to be active.
In 2014, Williamson and Hariri first showed that the presence of marks near the SLC6A4 gene can predict the way a person’s amygdala responds to threat. That study included both Williamson’s TAOS and Hariri’s DNS participants, but had looked at the chemical tags at a single point in time.
Looking at the changes in these markers over an extended time is a more powerful way to understand an individual’s risk for depression, said Hariri, who is also a member of the Duke Institute for Brain Sciences.
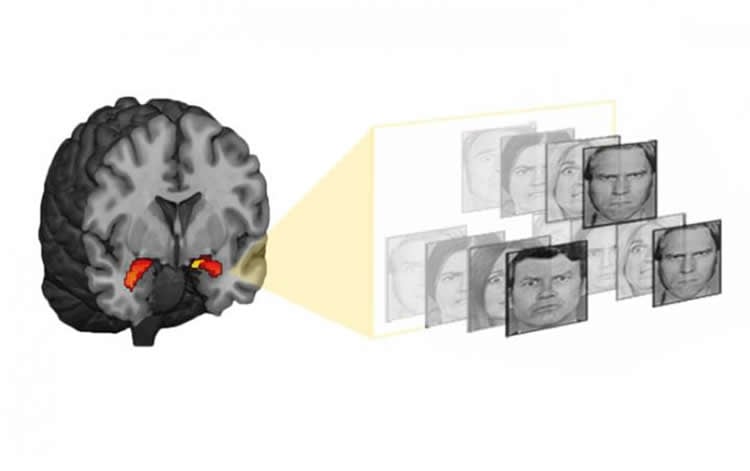
The team is now searching the genome for new markers that would predict depression. Ultimately, a panel of markers used in combination will lead to more accurate predictions, Swartz said.
They also hope to expand the age ranges of the study to include younger individuals and to continue following the TAOS participants into young adulthood.
“As they enter into young adulthood they are going to be experiencing more problems with depression or anxiety — or maybe substance abuse,” Hariri said. “The extent to which our measures of their genomes and brains earlier in their lives continue to predict their relative health is something that’s very important to know and very exciting for us to study.”
Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA016274), the Dielmann Family (MH087493), the National Institute on Drug Abuse grant (R01DA033369 and R01DA031579), the National Institute on Aging grant (R01AG049789), and the National Institutes of Health Center for the Study of Adolescent Risk and Resilience (P30DA023026).
Source: Karl Bates – Duke
Image Source: This NeuroscienceNews.com image is credited to Ahmad Hariri lab, Duke University.
Original Research: Abstract for “An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents” by J R Swartz, A R Hariri and D E Williamson in Molecular Psychiatry. Published online May 24 2016 doi:10.1038/MP.2016.82
[cbtabs][cbtab title=”MLA”]Duke. “Poverty Marks a Gene, Predicting Depression.” NeuroscienceNews. NeuroscienceNews, 24 May 2016.
<https://neurosciencenews.com/poverty-genetics-depression-4296/>.[/cbtab][cbtab title=”APA”]Duke. (2016, May 24). Poverty Marks a Gene, Predicting Depression. NeuroscienceNews. Retrieved May 24, 2016 from https://neurosciencenews.com/poverty-genetics-depression-4296/[/cbtab][cbtab title=”Chicago”]Duke. “Poverty Marks a Gene, Predicting Depression.” https://neurosciencenews.com/poverty-genetics-depression-4296/ (accessed May 24, 2016).[/cbtab][/cbtabs]
Abstract
An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents
Identifying biological mechanisms through which the experience of adversity emerges as individual risk for mental illness is an important step toward developing strategies for personalized treatment and, ultimately, prevention. Preclinical studies have identified epigenetic modification of gene expression as one such mechanism. Recent clinical studies have suggested that epigenetic modification, particularly methylation of gene regulatory regions, also acts to shape human brain function associated with risk for mental illness. However, it is not yet clear whether differential gene methylation as a function of adversity contributes to the emergence of individual risk for mental illness. Using prospective longitudinal epigenetic, neuroimaging and behavioral data from 132 adolescents, we demonstrate that changes in gene methylation associated with lower socioeconomic status (SES) predict changes in risk-related brain function. Specifically, we find that lower SES during adolescence is associated with an increase in methylation of the proximal promoter of the serotonin transporter gene, which predicts greater increases in threat-related amygdala reactivity. We subsequently demonstrate that greater increases in amygdala reactivity moderate the association between a positive family history for depression and the later manifestation of depressive symptoms. These initial results suggest a specific biological mechanism through which adversity contributes to altered brain function, which in turn moderates the emergence of general liability as individual risk for mental illness. If replicated, this prospective pathway may represent a novel target biomarker for intervention and prevention among high-risk individuals.
“An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents” by J R Swartz, A R Hariri and D E Williamson in Molecular Psychiatry. Published online May 24 2016 doi:10.1038/MP.2016.82






