Research conducted at Oregon Health and Science University Doernbecher Children’s Hospital challenges long-held belief that low blood flow to the premature brain necessarily kills brain cells.
Physician-scientists at Oregon Health and Science University Doernbecher Children’s Hospital are challenging the way pediatric neurologists think about brain injury in the pre-term infant. In a study published online in the Jan. 16 issue of Science Translational Medicine, the OHSU Doernbecher researchers report for the first time that low blood and oxygen flow to the developing brain does not, as previously thought, cause an irreversible loss of brain cells, but rather disrupts the cells’ ability to fully mature. This discovery opens up new avenues for potential therapies to promote regeneration and repair of the premature brain.
“As neurologists, we thought ischemia killed the neurons and that they were irreversibly lost from the brain. But this new data challenges that notion by showing that ischemia, or low blood flow to the brain, can alter the maturation of the neurons without causing the death of these cells. As a result, we can focus greater attention on developing the right interventions, at the right time early in development, to promote neurons to more fully mature and reduce the often serious impact of preterm birth. We now have a much more hopeful scenario,” said Stephen Back, M.D., Ph.D., lead investigator and professor of pediatrics and neurology in the Papé Family Pediatric Research Institute at OHSU Doernbecher Children’s Hospital.

Researchers at OHSU Doernbecher have conducted a number of studies in preterm fetal sheep to define how disturbances in brain blood flow lead to injury in the developing brain. Their findings have led to important advances in the care of critically ill newborn infants.
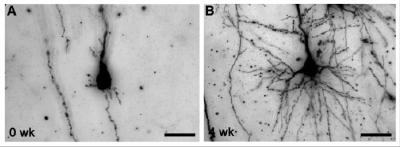
For this study, Back and colleagues used pioneering new MRI studies that allow injury to the developing brain to be identified much earlier than previously feasible. They looked at the cerebral cortex, or “thinking” part of the brain, which controls the complex tasks involved with learning, attention and social behaviors that are frequently impaired in children who survive preterm birth. Specifically, they observed how brain injury in the cerebral cortex of fetal sheep evolved over one month and found no evidence that cells were dying or being lost. They did notice, however, that more brain cells were packed into a smaller volume of brain tissue, which led to, upon further examination, the discovery that the brain cells weren’t fully mature.

In a related study published in the same online issue of Science Translational Medicine, investigators at The Hospital for Sick Children and the University of Toronto studied 95 premature infants using MRI and found that impaired growth of the infants was the strongest predictor of the MRI abnormalities, suggesting that interventions to improve infant nutrition and growth may lead to improved cortical development.
“I believe these studies provide hope for the future for preterm babies with brain injury, because our findings suggest that neurons are not being permanently lost from the human cerebral cortex due to ischemia. This raises the possibility that neurodevelopmental enrichment — or perhaps improved early infant nutrition — as suggested by the companion paper, might make a difference in terms of improved cognitive outcome,” Back said.
“Together, these studies challenge the conventional wisdom that preterm birth is associated with a loss of cortical neurons. This finding may change the way neurologists think about diagnosing and treating children born prematurely,” said Jill Morris, Ph.D., a program director at the National Institute’s of Health’s National Institute Neurological Disorders and Stroke.
More than 65,000 premature babies are born in the United States each year. Children who survive preterm birth commonly suffer from a wide range of life-long disabilities, including impaired walking due to cerebral palsy. Currently, children have a 10 times greater risk of acquiring cerebral palsy than of being diagnosed with cancer. By the time they reach school age, between 25 and 50 percent of children born prematurely are also identified with a wide range of learning disabilities, social impairment and attention deficit disorders.

Notes about this neurodevelopment research
Funding: This study was supported by National Institutes of Health grants P51RR000163 and NCRR P51 RR000113; National Institute of Neurological Disorders and Stroke grants 1RO1NS054044 and R37NS045737, R37NS045737-06S1/06S2 and 1F30NS066704; a Bugher Award from the American Heart Association; March of Dimes Birth Defects Foundation; and a Heubner Family Neurobiology of Disease Postdoctoral Fellowship.
Contact: Tamara Hargens-Bradley – Oregon Health and Science University
Source: Oregon Health and Science University press release
Image Sources: Images used in this article were adapted from the OHSU press release. Credit is given to Oregon Health & Science University.
Original Research: Abstract for “Prenatal Cerebral Ischemia Disrupts MRI-Defined Cortical Microstructure Through Disturbances in Neuronal Arborization” by Justin M. Dean, Evelyn McClendon, Kelly Hansen, Aryan Azimi-Zonooz, Kevin Chen, Art Riddle, Xi Gong, Elica Sharifnia, Matthew Hagen, Tahir Ahmad, Lindsey A. Leigland, A. Roger Hohimer, Christopher D. Kroenke, and Stephen A. Back in Science Translational Medicine 16 January 2013 VOL 5, Issue 168 doi: 10.1126/scitranslmed.3004669