Summary: RIKEN researchers discover deprivation of omega 3 and omega 6 fatty acids during pregnancy can alter gene expression, increasing the risk of schizophrenia in the offspring.
Source: RIKEN.
Researchers at the RIKEN Brain Science Institute in Japan have discovered a process through which changes in nutrition during early mouse pregnancy lead to offspring that develop schizophrenic-like symptoms as adults. Published in Translational Psychiatry, the study shows how deprivation of two polyunsaturated fatty acids during early gestation can have long lasting effects on offspring through specific epigenetic changes in gene expression.
Harmful conditions during pregnancy are known to affect the health of offspring, even resulting in adult-onset diseases. This concept–the Development Origin of Health and Disease (DOHaD)–can explain why rates of schizophrenia have been observed to double after famines. In order to develop effective treatments for schizophrenia, Takeo Yoshikawa and his team at RIKEN are researching exactly how malnutrition during early development changes the brain.
First author Motoko Maekawa notes that, “our work is the first in the field of psychiatry to identify a molecular cascade that links nutritional environment to disease risk in the context of the DOHaD paradigm.”
The first step to figuring out the “how”, was to determine the most likely nutrient whose deficiency is related to schizophrenia. Among several candidates that have been linked to schizophrenia, the team chose to study two specific polyunsaturated fatty acids–the omega-3 fatty acid DHA and the omega-6 fatty acid AA–because they are abundant in the brain and are known to be related to brain development. The team tested their theory by depriving pregnant mice of DHA and AA and testing whether their adult offspring shared characteristics exhibited by people with schizophrenia.
People in the early stages of schizophrenia have several common symptoms, including low levels of motivation, depression, impaired memory, and abnormal brain function in the prefrontal cortex. The adult mice whose mothers were deprived of DHA and AA showed similar symptoms.
As dysfunction in the prefrontal cortex is a hallmark of schizophrenia, the team next looked at how DHA/AA deprivation affects gene expression in that part of the brain. Among the hundreds of affected genes, they found a group of genes downregulated in people with schizophrenia that were also downregulated in the affected mice. These genes are related to oligodendrocytes, cells in the brain that surround neurons and help the transmission of signals in the brain. Additionally, expression of genes affecting the GABA neurotransmitter system were altered in ways that mimicked findings from the postmortem brains of people with schizophrenia.
Gene expression can be controlled by a certain class of proteins called nuclear receptors that attach to DNA and initiate the transcription process that builds proteins from the DNA code. When the team conducted further analysis of the fatty-acid deprived mice, they found several nuclear receptor genes related to fatty acids had been downregulated. It turns out that the abnormal expression of oligodendrocyte-related genes could be traced directly back to the low expression of these nuclear receptors, which in turn could be traced back to higher levels of DNA methylation, a common way to regulate gene expression. In this way, the altered diet succeeded in creating long-lasting changes in gene expression.
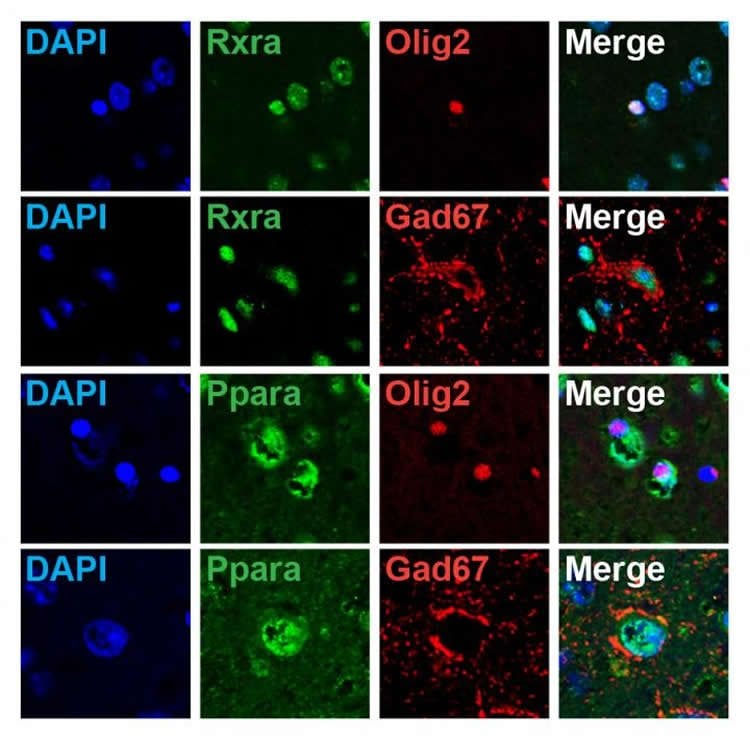
Once they knew which nuclear receptor genes were downregulated, the team could think about how to reverse the process. When they gave mice a drug that acts on RXR nuclear receptors, they found that oligodendrocyte- and GABA-related genes were upregulated, and that some of the abnormal motor behavior was reduced. “This was evidence that drugs acting on nuclear receptors can be a new therapy for schizophrenia,” says Maekawa.
Knowing the genes responsible also gave the team a target to investigate in people with schizophrenia. Analysis of hair follicles from two separate populations of patients with schizophrenia showed that they too exhibited reduced expression of the same nuclear receptor genes. “The next step,” explains Maekawa is to test the effectiveness of drugs that target these nuclear receptors in patients with schizophrenia, and to investigate how nuclear receptors regulate the function of oligodendrocytes and GABAergic neurons to prevent the development of schizophrenic pathophysiology.”
Source: Adam Phillips – RIKEN
Image Source: NeuroscienceNews.com image is credited to RIKEN.
Original Research: Full open access research for “Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes” by M Maekawa, A Watanabe, Y Iwayama, T Kimura, K Hamazaki, S Balan, H Ohba, Y Hisano, Y Nozaki, T Ohnishi, M Toyoshima, C Shimamoto, K Iwamoto, M Bundo, N Osumi, E Takahashi, A Takashima & T Yoshikawa in Translational Psychiatry. Published online
September 5 2017 doi:10.1038/tp.2017.182
[cbtabs][cbtab title=”MLA”]RIKEN “Prenatal Lack of Omegas 3 and 6 Linked to Schizophrenia Symptoms as Adults: Mouse Study.” NeuroscienceNews. NeuroscienceNews,5 September 2017.
<https://neurosciencenews.com/omega-3-6-pregnancy-schizophrenia-7416/>.[/cbtab][cbtab title=”APA”]RIKEN (2017, September 5). Prenatal Lack of Omegas 3 and 6 Linked to Schizophrenia Symptoms as Adults: Mouse Study. NeuroscienceNew. Retrieved September 5, 2017 from https://neurosciencenews.com/omega-3-6-pregnancy-schizophrenia-7416/[/cbtab][cbtab title=”Chicago”]RIKEN “Prenatal Lack of Omegas 3 and 6 Linked to Schizophrenia Symptoms as Adults: Mouse Study.” https://neurosciencenews.com/omega-3-6-pregnancy-schizophrenia-7416/ (accessed September 5, 2017).[/cbtab][/cbtabs]
Abstract
Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes
The risk of schizophrenia is increased in offspring whose mothers experience malnutrition during pregnancy. Polyunsaturated fatty acids (PUFAs) are dietary components that are crucial for the structural and functional integrity of neural cells, and PUFA deficiency has been shown to be a risk factor for schizophrenia. Here, we show that gestational and early postnatal dietary deprivation of two PUFAs—arachidonic acid (AA) and docosahexaenoic acid (DHA)—elicited schizophrenia-like phenotypes in mouse offspring at adulthood. In the PUFA-deprived mouse group, we observed lower motivation and higher sensitivity to a hallucinogenic drug resembling the prodromal symptoms in schizophrenia. Furthermore, a working-memory task-evoked hyper-neuronal activity in the medial prefrontal cortex was also observed, along with the downregulation of genes in the prefrontal cortex involved in oligodendrocyte integrity and the gamma-aminobutyric acid (GABA)-ergic system. Regulation of these genes was mediated by the nuclear receptor genes Rxr and Ppar, whose promoters were hyper-methylated by the deprivation of dietary AA and DHA. In addition, the RXR agonist bexarotene upregulated oligodendrocyte- and GABA-related gene expression and suppressed the sensitivity of mice to the hallucinogenic drug. Notably, the expression of these nuclear receptor genes were also downregulated in hair-follicle cells from schizophrenia patients. These results suggest that PUFA deficiency during the early neurodevelopmental period in mice could model the prodromal state of schizophrenia through changes in the epigenetic regulation of nuclear receptor genes.
“Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes” by M Maekawa, A Watanabe, Y Iwayama, T Kimura, K Hamazaki, S Balan, H Ohba, Y Hisano, Y Nozaki, T Ohnishi, M Toyoshima, C Shimamoto, K Iwamoto, M Bundo, N Osumi, E Takahashi, A Takashima & T Yoshikawa in Translational Psychiatry. Published online
September 5 2017 doi:10.1038/tp.2017.182






