Scientists at The University of Manchester have discovered a new genetic syndrome of obesity, over-eating, mental and behavioural problems in six families, from across the world.
Dr Siddharth Banka, Clinical Senior Lecturer at the Manchester Centre for Genomic Medicine, who led the study, explained: “Our team has identified that this new syndrome is caused by a small deletion on chromosome 6 that affects the function of hypothalamus, a region of the brain that plays a number of important roles in the body.”
Working in collaboration with Dr Eric Glasgow of the Georgetown University Medical Center in Washington D.C., the two teams used zebrafish models to study the consequences of chromosome 6 deletion and showed that the deletion has an effect on specific cells in the hypothalamus that produce a hormone called oxytocin.
This explains why sufferers are often severely obese, find it difficult to control their appetites and are prone to mood swings and being withdrawn.
The study, published in the latest issue of the American Journal of Human Genetics represents an important step in our understanding of how the hypothalamus and oxytocin control appetite and behaviour. Dr Banka, who is also a Consultant Clinical Geneticist at Saint Mary’s Hospital, said: “This finding demonstrates the power of human genetic study of rare conditions.”
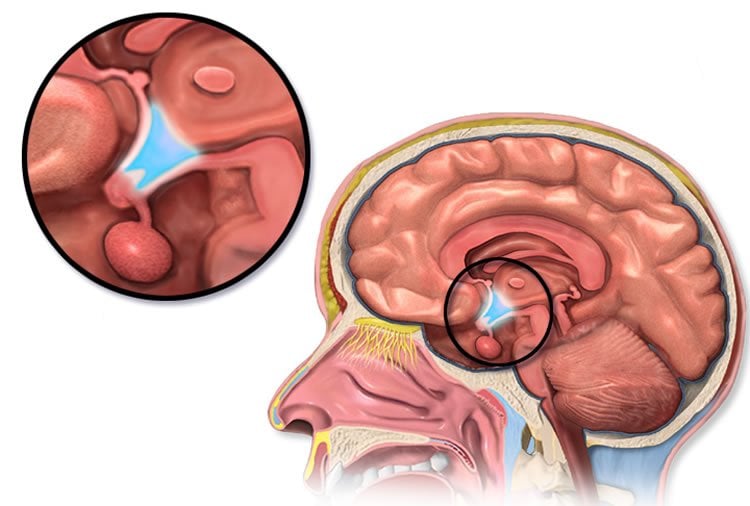
Although at an early stage, the researchers hope that the findings will help form part of a picture of how the hypothalamus works and may lead to new treatments in the future.
Source: University of Manchester
Image Source: The image is credited to BruceBlaus and is licensed CC BY 3.0
Original Research: Abstract for “Small 6q16.1 Deletions Encompassing POU3F2 Cause Susceptibility to Obesity and Variable Developmental Delay with Intellectual Disability” by Paul R. Kasher, Katherine E. Schertz, Megan Thomas, Adam Jackson, Silvia Annunziata, María J. Ballesta-Martinez, Philippe M. Campeau, Peter E. Clayton, Jennifer L. Eaton, Tiziana Granata, Encarna Guillén-Navarro, Cristina Hernando, Caroline E. Laverriere, Agne Liedén, Olaya Villa-Marcos, Meriel McEntagart, Ann Nordgren, Chiara Pantaleoni, Céline Pebrel-Richard, Catherine Sarret, Francesca L. Sciacca, Ronnie Wright, Bronwyn Kerr, Eric Glasgow, and Siddharth Banka in American Journal of Human Genetics. Published online December 15 2015 doi:10.1016/j.ajhg.2015.12.014
Abstract
Small 6q16.1 Deletions Encompassing POU3F2 Cause Susceptibility to Obesity and Variable Developmental Delay with Intellectual Disability
Genetic studies of intellectual disability and identification of monogenic causes of obesity in humans have made immense contribution toward the understanding of the brain and control of body mass. The leptin > melanocortin > SIM1 pathway is dysregulated in multiple monogenic human obesity syndromes but its downstream targets are still unknown. In ten individuals from six families, with overlapping 6q16.1 deletions, we describe a disorder of variable developmental delay, intellectual disability, and susceptibility to obesity and hyperphagia. The 6q16.1 deletions segregated with the phenotype in multiplex families and were shown to be de novo in four families, and there was dramatic phenotypic overlap among affected individuals who were independently ascertained without bias from clinical features. Analysis of the deletions revealed a ∼350 kb critical region on chromosome 6q16.1 that encompasses a gene for proneuronal transcription factor POU3F2, which is important for hypothalamic development and function. Using morpholino and mutant zebrafish models, we show that POU3F2 lies downstream of SIM1 and controls oxytocin expression in the hypothalamic neuroendocrine preoptic area. We show that this finding is consistent with the expression patterns of POU3F2 and related genes in the human brain. Our work helps to further delineate the neuro-endocrine control of energy balance/body mass and demonstrates that this molecular pathway is conserved across multiple species.
“Small 6q16.1 Deletions Encompassing POU3F2 Cause Susceptibility to Obesity and Variable Developmental Delay with Intellectual Disability” by Paul R. Kasher, Katherine E. Schertz, Megan Thomas, Adam Jackson, Silvia Annunziata, María J. Ballesta-Martinez, Philippe M. Campeau, Peter E. Clayton, Jennifer L. Eaton, Tiziana Granata, Encarna Guillén-Navarro, Cristina Hernando, Caroline E. Laverriere, Agne Liedén, Olaya Villa-Marcos, Meriel McEntagart, Ann Nordgren, Chiara Pantaleoni, Céline Pebrel-Richard, Catherine Sarret, Francesca L. Sciacca, Ronnie Wright, Bronwyn Kerr, Eric Glasgow, and Siddharth Banka in American Journal of Human Genetics. Published online December 15 2015 doi:10.1016/j.ajhg.2015.12.014






