The only drug currently approved for treatment of stroke’s crippling effects shows promise, when administered as a nasal spray, to help heal similar damage in less severe forms of traumatic brain injury.
In the first examination of its kind, researchers Ye Xiong, Ph.D, Zhongwu Liu, Ph.D., and Michael Chopp, Ph.D., Scientific Director of the Henry Ford Neuroscience Institute, found in animal studies that the brain’s limited ability to repair itself after trauma can be enhanced when treated with the drug tPA, or tissue plasminogen activator.
“Using this novel procedure in our earlier stroke studies, we found significant improvement in neurological function,” said Michael Chopp, Ph.D., scientific director of the Henry Ford Neuroscience Institute. “So we essentially repeated the experiment on lab rats with subacute traumatic brain injury, and with similar remarkable results. “As in stroke treated intra-nasally with tPA, our subjects showed greatly improved functional outcome and rewiring of the cortical spinal tract.”
The new study was recently published in the Public Library of Science’s peer-reviewed online journal PLOS ONE.
Commonly called a “clot-buster,” tPA is the only FDA-approved treatment for acute ischemic stroke.
Acute ischemic stroke occurs when oxygen-rich blood flow to the brain is blocked by a clot. Resulting damage to oxygen-starved brain cells can lead to physical impairment, mental disabilities and sometimes death.
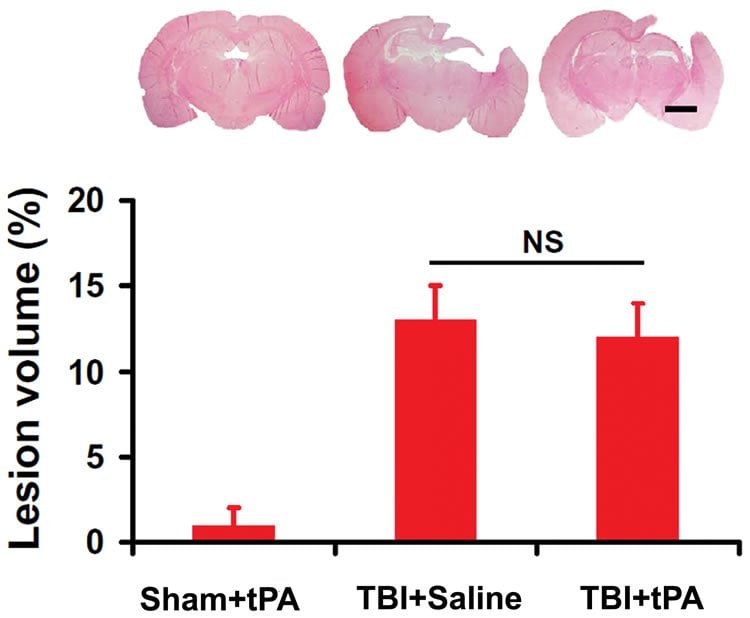
In the case of traumatic brain injury, damage is due to a violent blow or other external assault. It has been known for some time that stroke damage can be reduced if tPA is given intravenously within 4.5 hours. But tPA administered through the bloodstream also has potentially harmful side effects, including swelling of the brain and hemorrhage.
More recently, however, Henry Ford researchers found that the effective treatment window could be extended to as much as two weeks for lab rats dosed with tPA in a nasal spray, while avoiding the harmful side effects of intravenous injection.
Although scientists do not yet fully understand how it works, earlier research has shown that drugs administered through the nose directly target both the brain and spinal cord. Traumatic brain injury is a leading cause of death and disability throughout the world.
While the new Henry Ford study offers hope of a drug treatment, so far no effective pharmacological therapy is available. These most recent findings suggest that tPA has the potential to be a noninvasive treatment for subacute traumatic brain injury, helping the brain restore function to damaged cells.
The researchers cautioned that further animal studies will be required to discover the best dose and the best time window for optimal intranasal treatment.
Funding: National Institute of Neurological Disorders and Stroke RO1 NS062002 (YX), and National Institute on Aging RO1 AG037506 (MC).
Contact: Dwight Angell – Henry Ford Health System
Source: Henry Ford Health System press release
Image Source: The image is credited to Meng et al./PLOS ONE and is adapted from the open access research paper
Original Research: Full open access research for “Subacute Intranasal Administration of Tissue Plasminogen Activator Promotes Neuroplasticity and Improves Functional Recovery following Traumatic Brain Injury in Rats” by Yuling Meng, Michael Chopp, Yanlu Zhang, Zhongwu Liu, Aaron An, Asim Mahmood, and Ye Xiong in PLOS ONE. Published online September 3 2014 doi:10.1371/journal.pone.0106238
Subacute Intranasal Administration of Tissue Plasminogen Activator Promotes Neuroplasticity and Improves Functional Recovery following Traumatic Brain Injury in Rats
Traumatic brain injury (TBI) is a major cause of death and long-term disability worldwide. To date, there are no effective pharmacological treatments for TBI. Recombinant human tissue plasminogen activator (tPA) is the effective drug for the treatment of acute ischemic stroke. In addition to its thrombolytic effect, tPA is also involved in neuroplasticity in the central nervous system. However, tPA has potential adverse side effects when administered intravenously including brain edema and hemorrhage. Here we report that tPA, administered by intranasal delivery during the subacute phase after TBI, provides therapeutic benefit. Animals with TBI were treated intranasally with saline or tPA initiated 7 days after TBI. Compared with saline treatment, subacute intranasal tPA treatment significantly 1) improved cognitive (Morris water maze test) and sensorimotor (footfault and modified neurological severity score) functional recovery in rats after TBI, 2) reduced the cortical stimulation threshold evoking ipsilateral forelimb movement, 3) enhanced neurogenesis in the dentate gyrus and axonal sprouting of the corticospinal tract originating from the contralesional cortex into the denervated side of the cervical gray matter, and 4) increased the level of mature brain-derived neurotrophic factor. Our data suggest that subacute intranasal tPA treatment improves functional recovery and promotes brain neurogenesis and spinal cord axonal sprouting after TBI, which may be mediated, at least in part, by tPA/plasmin-dependent maturation of brain-derived neurotrophic factor.
“Subacute Intranasal Administration of Tissue Plasminogen Activator Promotes Neuroplasticity and Improves Functional Recovery following Traumatic Brain Injury in Rats” by Yuling Meng, Michael Chopp, Yanlu Zhang, Zhongwu Liu, Aaron An, Asim Mahmood, and Ye Xiong in PLOS ONE, September 3 2014 doi:10.1371/journal.pone.0106238.






