Summary: Researchers transfer genes into muller glial cells to activate stem cells properties and cause them to reproduce.
Source: Yale.
Many diseases that lead to blindness, such as glaucoma and macular degeneration, are caused by the death of certain cells in the human retina that lack the ability to regenerate. But in species such as zebrafish these cells, known as Muller glial cells (MGs), do serve as retinal stem cells that are capable of generating new cells. In a new study, a research team led by Associate Professor of Ophthalmology Bo Chen investigated whether the regenerative power of cells in zebrafish could be recreated in mammals, specifically mice.
The research team transferred genes into MGs to activate the stem cell properties of these normally dormant cells, causing them to reproduce and make other types of retinal cells.
The strategy could be developed into a therapeutic tool, Chen said. “In the future we are hoping to manipulate these cells to replenish any lost retinal neurons, either in diseased or physically damaged retinas,” he noted. “Potentially, it’s a therapy to treat many different retinal degenerative diseases.”
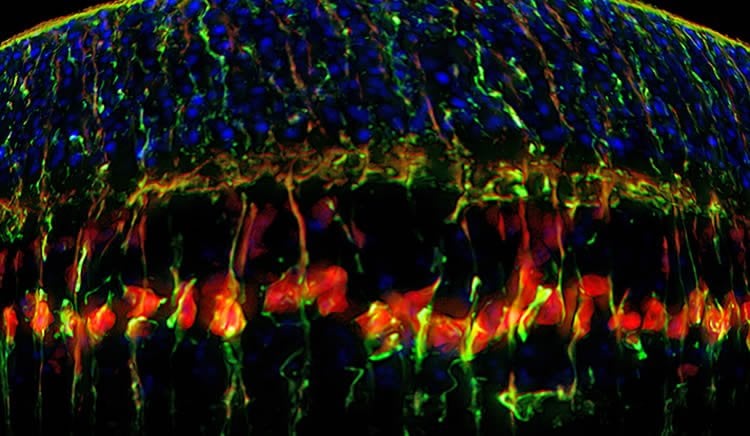
Source: Ziba Kashef – Yale
Image Source: This NeuroscienceNews.com image is credited to the researchers.
Original Research: Full open access research for “Wnt Regulates Proliferation and Neurogenic Potential of Müller Glial Cells via a Lin28/let-7 miRNA-Dependent Pathway in Adult Mammalian Retinas” by Kai Yao, Suo Qiu, Lin Tian, William D. Snider, John G. Flannery, David V. Schaffer, and Bo Chen in Cell Reports. Published online August 23 2016 doi:10.1016/j.celrep.2016.08.078
[cbtabs][cbtab title=”MLA”]Yale. “New Insight into Eye Disease.” NeuroscienceNews. NeuroscienceNews, 28 September 2016.
<https://neurosciencenews.com/muller-glia-cells-visual-neuroscience-5145/>.[/cbtab][cbtab title=”APA”]Yale. (2016, September 28). New Insight into Eye Disease. NeuroscienceNews. Retrieved September 28, 2016 from https://neurosciencenews.com/muller-glia-cells-visual-neuroscience-5145/[/cbtab][cbtab title=”Chicago”]Yale. “New Insight into Eye Disease.” https://neurosciencenews.com/muller-glia-cells-visual-neuroscience-5145/ (accessed September 28, 2016).[/cbtab][/cbtabs]
Abstract
Wnt Regulates Proliferation and Neurogenic Potential of Müller Glial Cells via a Lin28/let-7 miRNA-Dependent Pathway in Adult Mammalian Retinas
Highlights
•Retinal injury activates Wnt signaling in MGs
•Wnt-Lin28-let-7 miRNA signaling regulates MG proliferation
•Gene transfer of β-catenin or Lin28 stimulates MG proliferation without injury
•Cell-cycle-reactivated MGs show neurogenic potential
Summary
In cold-blooded vertebrates such as zebrafish, Müller glial cells (MGs) readily proliferate to replenish lost retinal neurons. In mammals, however, MGs lack regenerative capability as they do not spontaneously re-enter the cell cycle unless the retina is injured. Here, we show that gene transfer of β-catenin in adult mouse retinas activates Wnt signaling and MG proliferation without retinal injury. Upstream of Wnt, deletion of GSK3β stabilizes β-catenin and activates MG proliferation. Downstream of Wnt, β-catenin binds to the Lin28 promoter and activates transcription. Deletion of Lin28 abolishes β-catenin-mediated effects on MG proliferation, and Lin28 gene transfer stimulates MG proliferation. We further demonstrate that let-7 miRNAs are critically involved in Wnt/Lin28-regulated MG proliferation. Intriguingly, a subset of cell-cycle-reactivated MGs express markers for amacrine cells. Together, these results reveal a key role of Wnt-Lin28-let7 miRNA signaling in regulating proliferation and neurogenic potential of MGs in the adult mammalian retina.
“Wnt Regulates Proliferation and Neurogenic Potential of Müller Glial Cells via a Lin28/let-7 miRNA-Dependent Pathway in Adult Mammalian Retinas” by Kai Yao, Suo Qiu, Lin Tian, William D. Snider, John G. Flannery, David V. Schaffer, and Bo Chen in Cell Reports. Published online August 23 2016 doi:10.1016/j.celrep.2016.08.078






