Summary: Researchers have created super-sniffer mice that have an increased ability to detect a specific odor.
Source: Cell Press.
Researchers at Hunter College, part of the City University of New York, have created super-sniffer mice that have an increased ability to detect a specific odor, according to a study published July 7 in Cell Reports. The mice, which can be tuned to have different levels of sensitivity to any smell by using mouse or human odor receptors, could be used as land-mine detectors or as the basis for novel disease sensors.
The technology, a transgenic approach to engineering the mouse genome, could also provide researchers with a way to study human odor receptors. “This is one of our five basic senses, yet we have almost no clue how odors are coded by the brain,” says lead investigator Paul Feinstein, an associate professor of biological sciences at Hunter. “It’s still a black box.”
The nature of the odor receptors was discovered in 1991, a Nobel Prize winning feat, but exactly how the olfactory system wires itself still isn’t well understood. The noses of mammals contain a collection of sensory neurons, each equipped with a single chemical sensor called a receptor that detects a specific odor. In mice, as in humans, each neuron selects only one receptor. Collectively, neurons choose an even distribution of receptors, so each of the thousand distinct receptors is represented in about 0.1 percent of neurons.
In an effort to understand the mechanism these neurons use to choose a specific receptor, Feinstein tinkered with the mouse genome. He introduced the DNA for an odor receptor gene transgenically, by injection into the nucleus of a fertilized egg cell. He also added an extra string of DNA to the gene sequence to see if it would alter the probability of the gene being chosen. After a few attempts, he found a string that, when copied four or more times, worked.
More copies of this extra string of DNA resulted in a series of super-sniffer mice with increasing numbers of neurons expressing the selected receptor, a well-characterized receptor that detects acetophenone, which has a sweet smell similar to jasmine. The mice still maintain a relatively even distribution of other odor receptors. “We don’t know how the neuron performs singular gene choice yet, but we can increase the probability of a given choice occurring,” says Feinstein.
In parallel, post-doctoral researcher Charlotte D’Hulst was trying to replace a mouse receptor gene with a human one. Even though such gene swapping is standard practice in other fields, it did not work. It wasn’t the first time researchers had been stymied by olfaction. Repeated attempts in the field to study odor receptors by growing them in cells in Petri dishes have also led to dead ends.
As a result, human olfaction receptors are poorly understood. “Without understanding how odors bind to receptors, people have no rational way of designing new odors,” says D’Hulst. “They also have no way of boosting the diminished smell capacity in patients with diseases such as Parkinson’s.”
So D’Hulst abandoned gene swapping and tried Feinstein’s transgenic super-sniffer technique to insert a human receptor gene into the mouse. It worked. “We have developed a system where we can study human odor receptors and finally determine how human odor coding works,” says Feinstein.
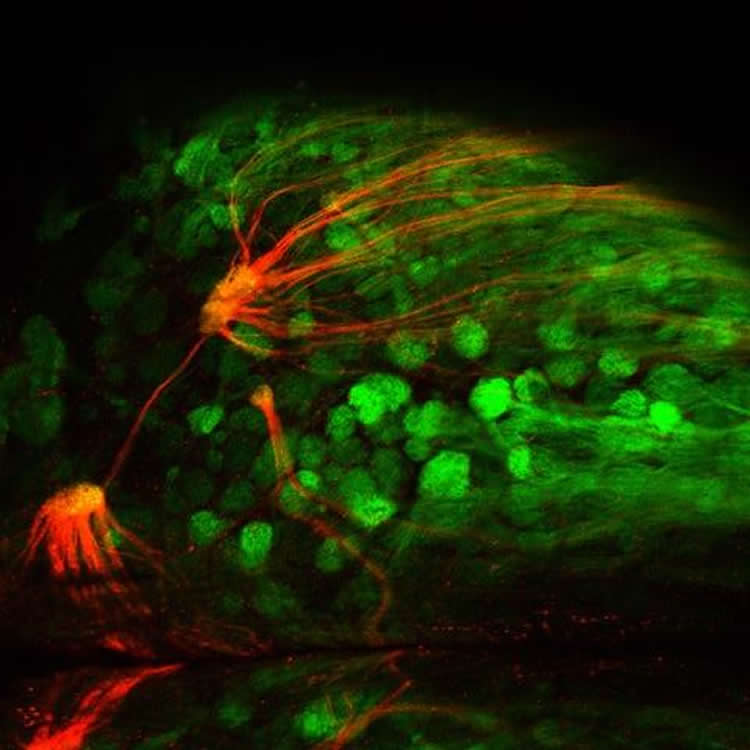
The team validated that the mice do indeed have an amplified sense of smell for the given receptor. They first used fluorescent imaging in live mice to trace the activation of the amplified odor receptor in response to the receptor’s corresponding odor. These tests gave visual confirmation that the receptors are functional and present in greater numbers than others.

In a standard behavioral test in which animals were trained to avoid an odor known to bind the transgenic receptor, the super-sniffer mice were able to detect the presence of this unpleasant odor in water at levels two orders of magnitude lower than those detectable by mice without super-sniffer abilities. “The animals could smell the odor better because of the increased presence of the receptor,” says D’Hulst.
The team is now working towards commercializing their technology and has founded a company called MouSensor, LLC. The Feinstein lab has received funding from the Department of Defense to develop super-sniffing rats that can be trained to detect TNT and potentially find land mines. The researchers also envision applications of the MouSensor for developing a type of nose-on-a-chip as a means of diagnosing disease using chemical detection profiling. “We have these millions-of-years-old receptors that are highly tuned to detect chemicals,” says Feinstein. “We think we can develop them into tools and use them to detect disease.”
Funding: This work was supported by the National Institutes of Health.
Source: Joseph Caputo – Cell Press
Image Source: This NeuroscienceNews.com images are credited to Paul Feinstein and D’Hulst et al./Cell Reports 2016.
Original Research: Full open access research for “MouSensor: A Versatile Genetic Platform to Create Super Sniffer Mice for Studying Human Odor Coding” by Charlotte D’Hulst, Raena B. Mina, Zachary Gershon, Sophie Jamet, Antonio Cerullo, Delia Tomoiaga, Li Bai, Leonardo Belluscio, Matthew E. Rogers, Yevgeniy Sirotin, and Paul Feinstein in Cell Reports. Published online July 7 2016 doi:10.1016/j.celrep.2016.06.047
[cbtabs][cbtab title=”MLA”]Cell Press. “Sniffing Out Danger: Super Sniffer Mice Could Be Used to Detect Land Mines.” NeuroscienceNews. NeuroscienceNews, 7 July 2016.
<https://neurosciencenews.com/mouse-olfaction-danger-4638/>.[/cbtab][cbtab title=”APA”]Cell Press. (2016, July 7). Sniffing Out Danger: Super Sniffer Mice Could Be Used to Detect Land Mines. NeuroscienceNew. Retrieved July 7, 2016 from https://neurosciencenews.com/mouse-olfaction-danger-4638/[/cbtab][cbtab title=”Chicago”]Cell Press. “Sniffing Out Danger: Super Sniffer Mice Could Be Used to Detect Land Mines.” https://neurosciencenews.com/mouse-olfaction-danger-4638/ (accessed July 7, 2016).[/cbtab][/cbtabs]
Abstract
MouSensor: A Versatile Genetic Platform to Create Super Sniffer Mice for Studying Human Odor Coding
Highlights
•Odorant receptor expression can be controlled using a 21-bp gene choice enhancer
•In vivo synaptopHluorin imaging shows functional glomerular activation in MouSensors
•MouSensors show lower specific odorant detection thresholds
•Human odorant receptors can be expressed using the MouSensor genetic platform
Summary
Typically, ∼0.1% of the total number of olfactory sensory neurons (OSNs) in the main olfactory epithelium express the same odorant receptor (OR) in a singular fashion and their axons coalesce into homotypic glomeruli in the olfactory bulb. Here, we have dramatically increased the total number of OSNs expressing specific cloned OR coding sequences by multimerizing a 21-bp sequence encompassing the predicted homeodomain binding site sequence, TAATGA, known to be essential in OR gene choice. Singular gene choice is maintained in these “MouSensors.” In vivo synaptopHluorin imaging of odor-induced responses by known M71 ligands shows functional glomerular activation in an M71 MouSensor. Moreover, a behavioral avoidance task demonstrates that specific odor detection thresholds are significantly decreased in multiple transgenic lines, expressing mouse or human ORs. We have developed a versatile platform to study gene choice and axon identity, to create biosensors with great translational potential, and to finally decode human olfaction.
“MouSensor: A Versatile Genetic Platform to Create Super Sniffer Mice for Studying Human Odor Coding” by Charlotte D’Hulst, Raena B. Mina, Zachary Gershon, Sophie Jamet, Antonio Cerullo, Delia Tomoiaga, Li Bai, Leonardo Belluscio, Matthew E. Rogers, Yevgeniy Sirotin, and Paul Feinstein in Cell Reports. Published online July 7 2016 doi:10.1016/j.celrep.2016.06.047






