Motor neuron connections are refined in the weeks before and just after birth, and they are crucial for normal development later, University of Queensland research suggests.
The discovery could lead to a better understanding of developmental disorders such as autism and epilepsy, say two School of Biomedical Sciences neuroscientists, Associate Professors Peter Noakes and Mark Bellingham.
“Motor neurons form a direct connection between the nervous system and the muscles,” Dr Bellingham said.
“It has been well established previously that it is vital that motor neurons do not become over-excited during critical foetal and neonatal periods.”
The neuroscientists made the discovery about the importance of the weeks before and after birth during research into the motor neurons that control breathing.
“This intricate balance during respiratory motor system development is particularly important because it’s vital that breathing begins at the moment of birth,” Dr Bellingham said.
“When the neural circuits are put together in foetal development, all neurotransmitters in the brain and spinal cord act as excitatory influences on motor neurons.
“Our research using a mouse model found that glycine prevented respiratory motor neurons from becoming ‘over-excited’ in the crucial final trimester of pregnancy when they are connecting with their target muscles.
“We also found the correct balance between motor neurons’ excitability and inhibition at this stage of foetal development was crucial for normal later life development in the mouse model.”
Dr Noakes said the study had far-reaching implications because the findings about respiratory motor neurons potentially could be applied to motor neurons controlling other types of movement.
“For humans and animals, a critical period when neural circuit connections are refined occurs not only in the last trimester but also immediately after birth,” he said.
“In the mouse model, excess connections in the brain are pruned away in the first three weeks after birth, leaving the connections that are necessary to elicit certain behaviours.
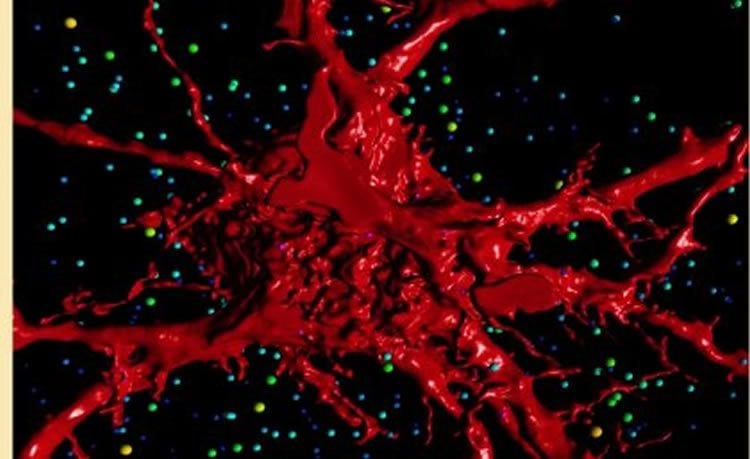
“If the excitation-inhibition balance is perturbed during this time, how the circuits are put together will be altered and once they become hard-wired, long term effects are possible.
“Previous studies have indicated that over-excitability in neural circuits at these critical stages can be linked to epilepsy, schizophrenia, autism, addiction and post-traumatic stress disorder in later life.”
Source: Peter Noakes – University of Queensland
Image Source: The image is adapted from the University of Queensland press release.
Original Research: Abstract for “Glycinergic Neurotransmission: A Potent Regulator of Embryonic Motor Neuron Dendritic Morphology and Synaptic Plasticity” by Matthew J. Fogarty, Refik Kanjhan, Mark C. Bellingham, and Peter G. Noakes in Journal of Neuroscience. Published online January 6 2016 doi:10.1523/JNEUROSCI.1576-15.2016
Abstract
Glycinergic Neurotransmission: A Potent Regulator of Embryonic Motor Neuron Dendritic Morphology and Synaptic Plasticity
Emerging evidence suggests that central synaptic inputs onto motor neurons (MNs) play an important role in developmental regulation of the final number of MNs and their muscle innervation for a particular motor pool. Here, we describe the effect of genetic deletion of glycinergic neurotransmission on single MN structure and on functional excitatory and inhibitory inputs to MNs. We measured synaptic currents in E18.5 hypoglossal MNs from brain slices using whole-cell patch-clamp recording, followed by dye-filling these same cells with Neurobiotin, to define their morphology by high-resolution confocal imaging and 3D reconstruction. We show that hypoglossal MNs of mice lacking gephyrin display increased dendritic arbor length and branching, increased spiny processes, decreased inhibitory neurotransmission, and increased excitatory neurotransmission. These findings suggest that central glycinergic synaptic activity plays a vital role in regulating MN morphology and glutamatergic central synaptic inputs during late embryonic development.
SIGNIFICANCE STATEMENT MNs within the brainstem and spinal cord are responsible for integrating a diverse array of synaptic inputs into discrete contractions of skeletal muscle to achieve coordinated behaviors, such as breathing, vocalization, and locomotion. The last trimester in utero is critical in neuromotor development, as this is when central and peripheral synaptic connections are made onto and from MNs. At this time-point, using transgenic mice with negligible glycinergic postsynaptic responses, we show that this deficiency leads to abnormally high excitatory neurotransmission and alters the dendritic architecture responsible for coherently integrating these inputs. This study compliments the emerging concept that neurodevelopmental disorders (including autism, epilepsy, and amyotrophic lateral sclerosis) are underpinned by synaptic dysfunction and therefore will be useful to neuroscientists and neurologists alike.
“Glycinergic Neurotransmission: A Potent Regulator of Embryonic Motor Neuron Dendritic Morphology and Synaptic Plasticity” by Matthew J. Fogarty, Refik Kanjhan, Mark C. Bellingham, and Peter G. Noakes in Journal of Neuroscience. Published online January 6 2016 doi:10.1523/JNEUROSCI.1576-15.2016







