Study suggests even prediabetes may cause nerve damage.
Results of a small study of people with tingling pain in their hands and feet have added to evidence that so-called prediabetes is more damaging to motor nerves than once believed, in a report on the study published online in JAMA Neurology on April 11.
Johns Hopkins neurologists say the study of patients with small fiber neuropathy showed unexpected deterioration over the entire length of sensory nerve fibers, rather than just at the longest ends first, which the investigators say defies the conventional wisdom of how nerves were thought to deteriorate.
Over the three-year course of the study of the 62 participants, 13 of them with prediabetes, the investigators found that generalized damage occurs in those with prediabetes, and that the precursor condition may be less benign than most clinicians appreciate.
“I liken small fiber neuropathy to the canary in the coal mine,” says senior author Michael Polydefkis, M.D., professor of neurology at the Johns Hopkins University School of Medicine and director of the Cutaneous Nerve Lab. “It signals the beginning of nerve deterioration that with time involves other types of nerve fibers and becomes more apparent and dramatically affects people’s quality of life. The results of this new study add urgency to the need for more screening of those with the condition and faster intervention.”
Small diameter nerve fibers reach out to the surface of the skin, and their damage is often marked by development of burning pain in the feet. But routine nerve tests, like nerve conduction, and routine examinations often fail to identify nerve damage because those measurements mostly assess injury to large diameter nerve fibers. The most common cause of small fiber neuropathy is diabetes, Polydefkis notes, but it can also be caused by lupus, HIV, Lyme disease, celiac disease or alcoholism.
In an effort to measure damage more accurately in small nerve fibers in people with small fiber neuropathy symptoms, Polydefkis and his team took small samples of skin — the size of a large freckle — from 52 patients diagnosed with small fiber neuropathy and from 10 healthy controls. Of the 52 patients enrolled in the study with small fiber neuropathy, 13 were considered to have prediabetes, 14 had type 2 diabetes, and 25 had normal blood sugar and an unknown cause of neuropathy. The participants ranged in age from their mid-40s to late 60s, and just less than half were female. Skin samples were taken from the ankle, the lower thigh near the knee and the upper thigh. Three years later, samples from the same area in the same group were taken for comparison.
Microscopic analysis of the skin samples was done to determine the density of small nerve fibers over time. According to the lead author of the study, Mohammad Khoshnoodi, M.D., assistant professor of neurology at Johns Hopkins, a lower density of fibers indicates more nerve damage.
Initially, he says, all patients with small fiber neuropathy had fewer nerve fibers at the test site on the ankle compared to the upper thigh, demonstrating more nerve damage the further down the leg measured. After three years, all three groups of those with small fiber neuropathy lost nerve fibers at the site tested in the ankle. But what surprised the researchers was that nerve fibers were lost at similar rates from the lower and upper thigh sites as well, a phenomenon that would not have been expected if the longest nerve fibers were the most vulnerable.
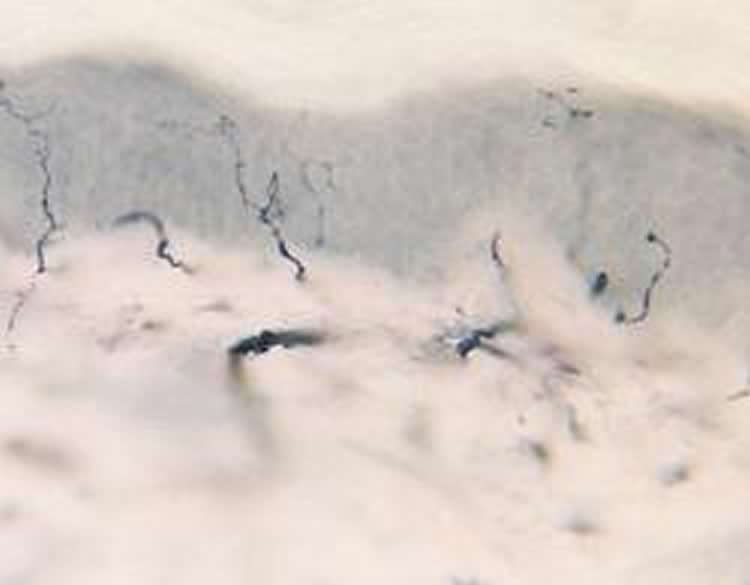
“We are all taught in medical school that the longest nerves degrade first, and we show that this isn’t always the case,” says Khoshnoodi.
Patients with prediabetes or diabetes had at least 50 percent fewer small nerve fibers in their ankles initially than those participants with an unknown cause for their small fiber neuropathy, indicating these patients started the study with more damage to their small nerve fibers.
The patients with prediabetes continued to have worsening damage to their small nerve fibers over the course of the study; they lost about 10 percent of their nerve fiber density each year at all sites tested along the leg. Patients with diabetes also lost similar rates of nerve fibers along the three sites of the leg.
“I expected that people with diabetes would do worse, but I didn’t really expect people with prediabetes to experience a similar rate of degradation of their small nerve fibers,” says Polydefkis.
Khoshnoodi cautions that the study was small, and that other factors in addition to high blood sugar, such as smoking, high blood pressure and high cholesterol, may also have contributed to the decline. Future studies that address these risk factors will need to be performed to determine if prediabetes is as debilitating to nerves as full-blown diabetes.
According to the National Institute of Neurological Disorders and Stroke, an estimated 20 million people in the U.S. have some form of peripheral neuropathy. About 50 percent of people with diabetes have neuropathy.
Other authors contributing to the study are Shaun Truelove, Ahmet Hoke and Andrew Mammen of The Johns Hopkins University and Ahmet Burakgazi from Virginia Tech.
Funding: The study was funded by a philanthropic gift from Mark Rubenstein.
Source: Vanessa McMains – Johns Hopkins Medicine
Image Credit: Image is credited to Michael Polydefkis.
Original Research: Abstract for “Longitudinal Assessment of Small Fiber Neuropathy: Evidence of a Non–Length-Dependent Distal Axonopathy” by Mohammad A. Khoshnoodi, MD; Shaun Truelove, BA; Ahmet Burakgazi, MD; Ahmet Hoke, MD, PhD; Andrew L. Mammen, MD, PhD; and Michael Polydefkis, MD, MHS in JAMA Neurology. Published online April 11 2016 doi:10.1001/jamaneurol.2016.0057
Abstract
Longitudinal Assessment of Small Fiber Neuropathy: Evidence of a Non–Length-Dependent Distal Axonopathy
Importance Few data are available on the natural history of small fiber neuropathy (SNF). Peripheral neuropathy typically follows a length-dependent pattern, leading us to hypothesize that patients with SFN would lose intraepidermal nerve fibers at the distal leg more quickly than at more proximal thigh sites.
Objective To compare the longitudinal rate and pattern of intraepidermal nerve fiber density (IENFD) change in idiopathic SFN (iSFN), impaired glucose tolerance–associated SFN (IGT-SFN), and diabetes mellitus–associated SFN (DM-SFN).
Design, Setting, and Participants In this longitudianl, case-control study, patients diagnosed as having SFN from January 1, 2002, through December 31, 2010, and age- and sex-matched controls underwent additional evaluation at tertiary outpatient neurology clinics. Participants and healthy controls were evaluated twice separated by at least 2 years. Participants underwent standardized examinations, nerve conduction, and skin biopsy at 3 sites along the leg. A linear mixed-effects model was used to compare rates of IENFD decrease between cause and biopsy site.
Main Outcomes and Measures We compared the rate of IENFD loss over time in subjects with iSFN, IGT-SFN and DM-SFN as well as the spatiotemporal pattern of IENF loss at different rostal-caudal sites along the leg.
Results Fifty-two participants (25 with iSFN, 13 with IGT-SFN, and 14 with DM-SFN) and 10 healthy controls were evaluated. Mean (SD) ages were 50.9 (12.9), 63.1 (10.4), and 61.6 (11.6) years for the iSFN, IGT-SFN, and DM-SFN groups, respectively. There were 12, 7, and 8 female patients and 13, 6, and 6 male patients in the iSFN, IGT-SFN, and DM-SFN groups, respectively. The mean follow-up time was 24.2, 26.7, and 38.8 months for those with iSFN, IGT-SFN, and DM-SFN, respectively, and 32 months for healthy controls. At baseline, mean (SE) for distal leg IENFD (6.48 [1.06]) was lower than distal thigh (13.32 [1.08]) and proximal thigh IENFD (19.98 [1.07]) (P = .001). In addition, IENFD was significantly lower in patients with DM-SFN and IGT-SFN compared with iSFN at all biopsy sites (P = .001). All 3 neuropathy groups had significant IENFD decrease at follow-up at all 3 sites (P = .002), whereas there was no change in the control group. The mean yearly rates of IENFD change over time at the distal leg, distal thigh, and proximal thigh irrespective of cause are −1.42, −1.59, and −2.8 fibers per millimeter, respectively. The mean slopes of IENFD change over time by cause regardless of biopsy site are −0.179, −0.164, and −0.198 for iSFN, IGT-SFN, and DM-SFN, respectively. No difference was found between SFN groups in the rate of decrease. The rate of IENFD decrease was similar at all 3 biopsy sites.
Conclusions and Relevance Similar rates of IENFD decrease irrespective of cause were observed. Epidermal nerve fibers were lost at similar rates in proximal and distal sites, suggesting that SFN is a non–length-dependent terminal axonopathy.
“Longitudinal Assessment of Small Fiber Neuropathy: Evidence of a Non–Length-Dependent Distal Axonopathy” by Mohammad A. Khoshnoodi, MD; Shaun Truelove, BA; Ahmet Burakgazi, MD; Ahmet Hoke, MD, PhD; Andrew L. Mammen, MD, PhD; and Michael Polydefkis, MD, MHS in JAMA Neurology. Published online April 11 2016 doi:10.1001/jamaneurol.2016.0057






