Find could be central to treating channel-related diseases such as cardiac arrhythmias, epilepsy and Parkinson’s.
A common protein plays a different role than previously thought in the opening and closing of channels that let ions flow in and out of our cells, researchers at Johns Hopkins report. Those channels are critical to life, as having the right concentrations of sodium and calcium ions in cells enables healthy brain communication, heart contraction and many other processes. The new study reveals that a form of calmodulin long thought to be dormant actually opens these channels wide. The finding is likely to bring new insight into disorders caused by faulty control of these channels, such as cardiac arrhythmias, epilepsy and Parkinson’s disease, the researchers say.
A report on the finding appears in the Oct. 23 issue of the journal Cell.
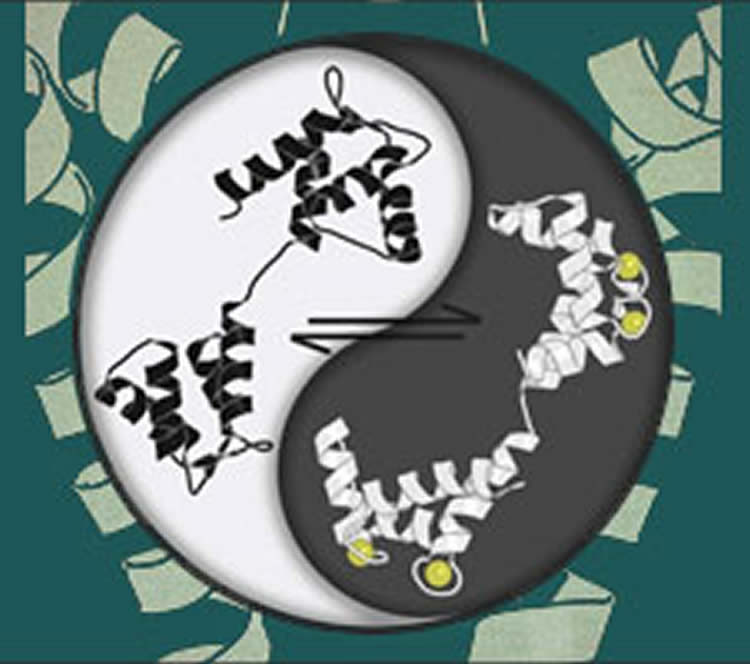
In the current model, explains David Yue, M.D., Ph.D. , a professor of biomedical engineering and neuroscience at the Johns Hopkins University School of Medicine, calmodulin can do little until it binds to calcium, which changes its shape and snaps it into action. The activated calmodulin can then bind to a specialized control lever inside calcium and sodium channels, which closes the channels.
The new study revises this viewpoint by devising ways to deliver surges of calcium-free calmodulin to channels. In so doing, “it can be seen that calcium-free calmodulin is in no way dormant, but instead markedly boosts the opening of calcium and sodium channels to begin with,” Yue says. When calcium binds to the “resident” calcium-free calmodulin on channels, this initial enhancement dissipates. “The two forms of calmodulin are both powerful, each imposing opposing actions that together maintain exquisite control, akin to the ‘yin-yang’ balance in Chinese philosophy,” Yue says. “This insight into how the calmodulin-controlled lever works could ultimately help in finding treatments for a plethora of conditions that stem from faulty ion channels.”
Notes about this neuroscience research
Other contributors to the paper were Paul J. Adams, Manu Ben-Johny, Ivy E. Dick and Takanari Inoue, all of The Johns Hopkins University.
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (grant numbers R01NS085074 and R01NS073874), the National Heart, Lung and Blood Institute (grant number R37HL076795), the National Institute of Mental Health (grant number F31MH88109) and Parkinson Society Canada.
Contact: Shawna Williams – Johns Hopkins Medicine
Source: Johns Hopkins Medicine press release
Image Source: The image is credited to Manu Ben-Johny and David Yue/Johns Hopkins Medicine and is adapted from the press release
Original Research: Abstract for “Apocalmodulin Itself Promotes Ion Channel Opening and Ca2+ Regulation” by Paul J. Adams, Manu Ben-Johny, Ivy E. Dick, Takanari Inoue, and David T. Yue in Cell. Published online September 26 2014 doi:10.1016/j.cell.2014.09.047






