Summary: A new study reports the chemotherapy drug cisplatin can kill sensory cells in the inner ear and cause permanent hearing loss.
Source: USC.
USC Stem Cell researchers show that cisplatin causes more acute hearing loss in mice with the equivalent of Cockayne syndrome.
The chemotherapy drug cisplatin can kill cancer, but it can also kill the sensory cells of the inner ear — causing permanent hearing loss. This hearing loss is likely to be more severe in individuals with Cockayne syndrome, according to a new study in The Journal of Neuroscience.
In the study, USC Stem Cell researchers Robert Rainey and Sum-yan Ng, from the laboratory of Neil Segil, demonstrated that cisplatin causes more acute hearing loss in mice with the equivalent of Cockayne syndrome.
In humans, Cockayne syndrome can cause hearing loss as well as eye abnormalities, shortness, skeletal deformities, microcephaly, nervous system underdevelopment, an appearance of premature aging and sun sensitivity. In another study published in the journal last year, the researchers established a new strain of mouse that models Cockyane syndrome-associated hearing loss.
The disorder results from mutations in one of two genes — called Csa and Csb — involved in repairing DNA damage. Cells can sustain DNA damage from environmental stresses ranging from the sun’s ultraviolet radiation to toxic chemicals such as chemotherapy drugs.
An effective chemotherapy drug
Like similar chemotherapy drugs, cisplatin damages the DNA in cells, interfering with their ability to proliferate. This interference is expected to have the most pronounced effect on the most proliferative cells, such as cancer cells, and the least effect on non-dividing cells, such as the sensory cells of the inner ear. However, in practice, cisplatin causes significant death of both quickly dividing cancer cells and the non-dividing sensory cells of the inner ear — making it an effective chemotherapy drug with a common side effect, severe hearing loss.
Young children undergoing cisplatin chemotherapy appear to be particularly vulnerable, and they consequently experience developmental delays as a result of early hearing loss.
Like humans with Cockayne syndrome, mice with mutations in Csa and Csb can’t efficiently repair DNA damage, leaving them particularly vulnerable to permanent hearing loss from cisplatin. In the study, mice with the Csa mutation fared somewhat worse than mice with the Csb mutation.
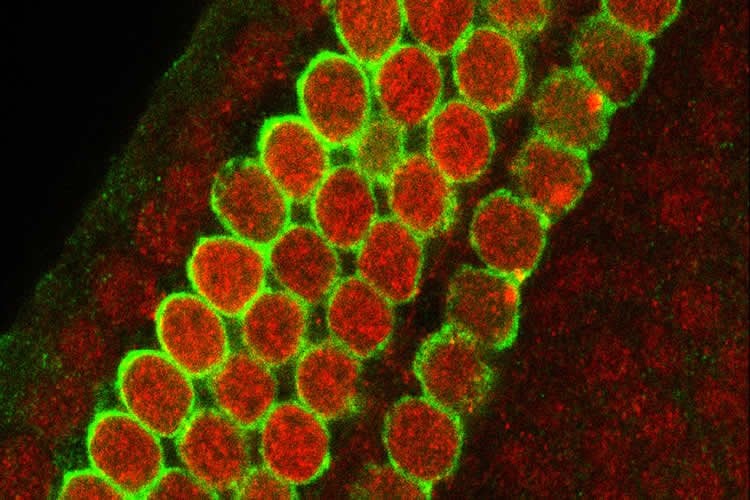
Both mutations interfere with what is known as transcription-coupled DNA repair, or TCR. While there are many different ways that cells can repair DNA damage, TCR appears to play a particularly important role in protecting the sensory cells of the inner ear from cisplatin. Variation between individuals in the effectiveness of TCR may help explain the differing susceptibility to hearing loss due to environmental stress and aging.
“Our cells have several biochemical pathways that they use to repair DNA. Our findings suggest that one particular pathway, transcription-coupled DNA repair, is a major force for protecting the cells of the inner ear from cisplatin,” Segil said. “This leaves patients with Cockayne syndrome particularly vulnerable to severe hearing loss as a side effect of taking this chemotherapy drug.”
Additional authors include Juan Llamas from USC and Gijsbertus T.J. van der Horst from the Erasmus University Medical Center in the Netherlands.
Funding: Funding came from the National Institutes of Health’s Ruth L. Kirschstein National Research Service Award (F32DC010125), an NIH grant (R01DC007173) and the Sidgmore Family Foundation.
Source: Cristy Lytal – USC
Image Source: This NeuroscienceNews.com image is credited to Sigil Lab.
Original Research: Abstract for “Mutations in Cockayne Syndrome-Associated Genes (Csa and Csb) Predispose to Cisplatin-Induced Hearing Loss in Mice” by Robert N. Rainey, Sum-yan Ng, Juan Llamas, Gijsbertus T. J. van der Horst, and Neil Segil in Journal of Neuroscience. Published online April 27 2016 doi:10.1523/JNEUROSCI.3890-15.2016
[cbtabs][cbtab title=”MLA”]USC. “Key Mutations May Worsen Hearing Loss From Chemotherapy Drug.” NeuroscienceNews. NeuroscienceNews, 22 May 2016.
<https://neurosciencenews.com/cisplatin-hearing-loss-4269/>.[/cbtab][cbtab title=”APA”]USC. (2016, May 22). Key Mutations May Worsen Hearing Loss From Chemotherapy Drug. NeuroscienceNews. Retrieved May 22, 2016 from https://neurosciencenews.com/cisplatin-hearing-loss-4269/[/cbtab][cbtab title=”Chicago”]USC. “Key Mutations May Worsen Hearing Loss From Chemotherapy Drug.” NeuroscienceNews.
https://neurosciencenews.com/cisplatin-hearing-loss-4269/ (accessed May 22, 2016).[/cbtab][/cbtabs]
Abstract
Mutations in Cockayne Syndrome-Associated Genes (Csa and Csb) Predispose to Cisplatin-Induced Hearing Loss in Mice
Cisplatin is a common and effective chemotherapeutic agent, yet it often causes permanent hearing loss as a result of sensory hair cell death. The causes of sensitivity to DNA-damaging agents in nondividing cell populations, such as cochlear hair and supporting cells, are poorly understood, as are the specific DNA repair pathways that protect these cells. Nucleotide excision repair (NER) is a conserved and versatile DNA repair pathway for many DNA-distorting lesions, including cisplatin-DNA adducts. Progressive sensorineural hearing loss is observed in a subset of NER-associated DNA repair disorders including Cockayne syndrome and some forms of xeroderma pigmentosum. We investigated whether either of the two overlapping branches that encompass NER, transcription-coupled repair or global genome repair, which are implicated in Cockayne syndrome and xeroderma pigmentosum group C, respectively, modulates cisplatin-induced hearing loss and cell death in the organ of Corti, the auditory sensory epithelium of mammals. We report that cochlear hair cells and supporting cells in transcription-coupled repair-deficient Cockayne syndrome group A (Csa−/−) and group B (Csb−/−) mice are hypersensitive to cisplatin, in contrast to global genome repair-deficient Xpc−/− mice, both in vitro and in vivo. We show that sensory hair cells in Csa−/− and Csb−/− mice fail to remove cisplatin-DNA adducts efficiently in vitro; and unlike Xpc−/− mice, Csa−/− and Csb−/− mice lose hearing and manifest outer hair cell degeneration after systemic cisplatin treatment. Our results demonstrate that Csa and Csb deficiencies predispose to cisplatin-induced hearing loss and hair/supporting cell damage in the mammalian organ of Corti, and emphasize the importance of transcription-coupled DNA repair in the protection against cisplatin ototoxicity.
SIGNIFICANCE STATEMENT The utility of cisplatin in chemotherapy remains limited due to serious side effects, including sensorineural hearing loss. We show that mouse models of Cockayne syndrome, a progeroid disorder resulting from a defect in the transcription-coupled DNA repair (TCR) branch of nucleotide excision repair, are hypersensitive to cisplatin-induced hearing loss and sensory hair cell death in the organ of Corti, the mammalian auditory sensory epithelium. Our work indicates that Csa and Csb, two genes involved in TCR, are preferentially required to protect against cisplatin ototoxicity, relative to global genome repair-specific elements of nucleotide excision repair, and suggests that TCR is a major force maintaining DNA integrity in the cochlea. The Cockayne syndrome mice thus represent a model for testing the contribution of DNA repair mechanisms to cisplatin ototoxicity.
“Mutations in Cockayne Syndrome-Associated Genes (Csa and Csb) Predispose to Cisplatin-Induced Hearing Loss in Mice” by Robert N. Rainey, Sum-yan Ng, Juan Llamas, Gijsbertus T. J. van der Horst, and Neil Segil in Journal of Neuroscience. Published online April 27 2016 doi:10.1523/JNEUROSCI.3890-15.2016






