Scientists have been exploring the connection between tricyclic antidepressants and brain cancer since the early 2000s. There’s some evidence that the drugs can lower one’s risk for developing aggressive glioblastomas, but when given to patients after diagnosis in a small clinical trial, the antidepressants showed no effect as a treatment.
n a study appearing in Cancer Cell on September 24, Swiss researchers find that antidepressants work against brain cancer by excessively increasing tumor autophagy (a process that causes the Cancer Cells to eat themselves). The scientists next combined the antidepressants with blood thinners–also known to increase autophagy–as a treatment for mice with the first stages of human glioblastoma. Mouse lifespan doubled with the drug combination therapy, while either drug alone had no effect.
“It is exciting to envision that combining two relatively inexpensive and non-toxic classes of generic drugs holds promise to make a difference in the treatment of patients with lethal brain cancer,” says senior study author Douglas Hanahan, of the Swiss Federal Institute of Technology (EPFL). “However, it is presently unclear whether patients might benefit from this treatment. This new mechanism-based strategy to therapeutically target glioblastoma is provocative, but at an early stage of evaluation, and will require considerable follow-up to assess its potential.”
Mice received the combination therapy 5 days a week with 10-15 minute intervals between drugs. The antidepressant was given orally, and the other drug (the blood thinner or anti-coagulant) was injected. The data suggest that the drugs act synergistically by disrupting, in two different places, the biological pathway that controls the rate of autophagy–a cellular recycling system that at low levels enhances cell survival in stressful conditions. The two drugs work together to hyper-stimulate autophagy, causing the Cancer Cells to die.
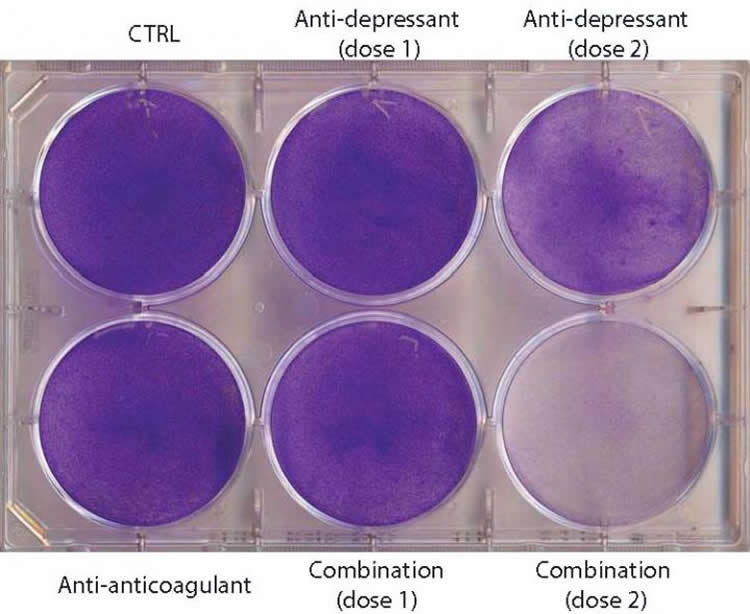
“Importantly, the combination therapy did not cure the mice; rather, it delayed disease progression and modestly extended their lifespan,” Hanahan says. “It seems likely that these drugs will need to be combined with other classes of anticancer drugs to have benefit in treating gliblastoma patients. One can also envision ‘co-clinical trials’ wherein experimental therapeutic trials in the mouse models of glioblastom are linked to analogous small proof-of-concept trials in GBM patients. Such trials may not be far off.”
Funding: This work was supported by grants from Fondation S.A.N.T.É. and the School of Life Sciences at EPFL.
Source: Joseph Caputo – Cell Press
Image Source: The image is credited to Douglas Hanahan
Original Research: Abstract for “Dual Targeting of the Autophagic Regulatory Circuitry in Gliomas with Repurposed Drugs Elicits Cell-Lethal Autophagy and Therapeutic Benefit” by Ksenya Shchors, Aristea Massaras, and Douglas Hanahan in Cancer Cell. Published online September 24 2015 doi:10.1016/j.ccell.2015.08.012
Abstract
Dual Targeting of the Autophagic Regulatory Circuitry in Gliomas with Repurposed Drugs Elicits Cell-Lethal Autophagy and Therapeutic Benefit
The associations of tricyclic antidepressants (TCAs) with reduced incidence of gliomas and elevated autophagy in glioma cells motivated investigation in mouse models of gliomagenesis. First, we established that imipramine, a TCA, increased autophagy and conveyed modest therapeutic benefit in tumor-bearing animals. Then we screened clinically approved agents suggested to affect autophagy for their ability to enhance imipramine-induced autophagy-associated cell death. The anticoagulant ticlopidine, which inhibits the purinergic receptor P2Y12, potentiated imipramine, elevating cAMP, a modulator of autophagy, reducing cell viability in culture, and increasing survival in glioma-bearing mice. Efficacy of the combination was obviated by knockdown of the autophagic regulatory gene ATG7, implicating cell-lethal autophagy. This seemingly innocuous combination of TCAs and P2Y12 inhibitors may have applicability for treating glioma.
“Dual Targeting of the Autophagic Regulatory Circuitry in Gliomas with Repurposed Drugs Elicits Cell-Lethal Autophagy and Therapeutic Benefit” by Ksenya Shchors, Aristea Massaras, and Douglas Hanahan in Cancer Cell. Published online September 24 2015 doi:10.1016/j.ccell.2015.08.012






