Summary: Targeting autophagy may help in the battle against ALS and other diseases that affect motor neurons, researchers report.
Source: University of Wurzburg.
Motor neurons are the nerves that send impulses to the muscles to generate movement. Damage of these neurons can cause very diverse diseases, for example spinal muscular atrophy in children or adult amyotrophic lateral sclerosis.
Both disorders are characterised by muscle atrophy, palsy and ultimately by functional loss of muscles. The respiratory muscles are affected, too. The diseases are presently incurable; but their progress can be slowed down with drugs.
Autophagy as a new target for drugs
“So far, the development of new drugs has focused on preventing cell death mechanisms and on breaking down the protein aggregates in the affected nerve cells,” Professor Michael Sendtner explains; he is the head of the Institute of Clinical Neurobiology of the Würzburg university hospital.
But now Sendtner with his colleague Patrick Lüningschrör and their team have identified another potential target for future drugs: It is the complex process of autophagy, as the researchers report in the journal Nature Communications. This process makes sure that the impulse transmission between motor neurons and muscles works permanently in normal function.
Aggregation of vesicles at synapses
The PLEKHG5 gene was the starting point for the new insight. Mutations in this gene are known to trigger various forms of motor neuron disorders. The Würzburg scientists have now found out that this gene is crucial for autophagy: It controls the degradation of the synaptic vesicles that contain the neurotransmitter acetylcholine and transport the excitation from the nerve to the muscles.
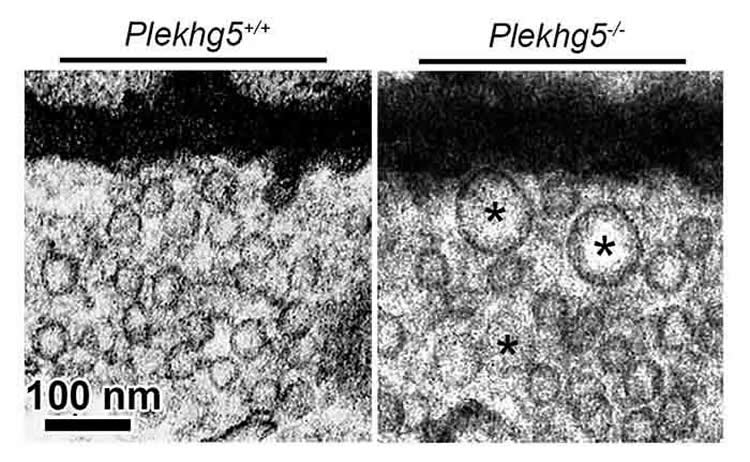
When deactivating the PLEKHG5 gene at single motor neurons in a cell culture, autophagy is reduced causing the synaptic vehicles to agglomerate. And in mice disabling this gene triggers a motor neuron disorder which also entails massive vesicle aggregation.
Consequences for drug development
“The findings deliver substantial evidence that dysfunctional autophagy plays a central role in the development of motor neuron diseases,” Sendtner says. He believes that this new finding will have to lead to a rethinking of the development of new drugs with a need for substances that prevent or reduce vesicle aggregation.
Source: Michael Sendtner – University of Wurzburg
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Peter Heimann.
Original Research: Full open access research for “Plekhg5-regulated autophagy of synaptic vesicles reveals a pathogenic mechanism in motoneuron disease” by Patrick Lüningschrör, Beyenech Binotti, Benjamin Dombert, Peter Heimann, Angel Perez-Lara, Carsten Slotta, Nadine Thau-Habermann, Cora R. von Collenberg, Franziska Karl, Markus Damme, Arie Horowitz, Isabelle Maystadt, Annette Füchtbauer, Ernst-Martin Füchtbauer, Sibylle Jablonka, Robert Blum, Nurcan Üçeyler, Susanne Petri, Barbara Kaltschmidt, Reinhard Jahn, Christian Kaltschmidt & Michael Sendtner in Nature Communications. Published online September 15 2017 doi:10.1038/s41467-017-00689-z
[cbtabs][cbtab title=”MLA”]University of Wurzburg “Synaptic Disorder.” NeuroscienceNews. NeuroscienceNews, 2 November 2017.
<https://neurosciencenews.com/autophagy-als-synapses-7856/>.[/cbtab][cbtab title=”APA”]University of Wurzburg (2017, November 2). Synaptic Disorder. NeuroscienceNews. Retrieved November 2, 2017 from https://neurosciencenews.com/autophagy-als-synapses-7856/[/cbtab][cbtab title=”Chicago”]University of Wurzburg “Synaptic Disorder.” https://neurosciencenews.com/autophagy-als-synapses-7856/ (accessed November 2, 2017).[/cbtab][/cbtabs]
Abstract
Plekhg5-regulated autophagy of synaptic vesicles reveals a pathogenic mechanism in motoneuron disease
Autophagy-mediated degradation of synaptic components maintains synaptic homeostasis but also constitutes a mechanism of neurodegeneration. It is unclear how autophagy of synaptic vesicles and components of presynaptic active zones is regulated. Here, we show that Pleckstrin homology containing family member 5 (Plekhg5) modulates autophagy of synaptic vesicles in axon terminals of motoneurons via its function as a guanine exchange factor for Rab26, a small GTPase that specifically directs synaptic vesicles to preautophagosomal structures. Plekhg5 gene inactivation in mice results in a late-onset motoneuron disease, characterized by degeneration of axon terminals. Plekhg5-depleted cultured motoneurons show defective axon growth and impaired autophagy of synaptic vesicles, which can be rescued by constitutively active Rab26. These findings define a mechanism for regulating autophagy in neurons that specifically targets synaptic vesicles. Disruption of this mechanism may contribute to the pathophysiology of several forms of motoneuron disease.
“Plekhg5-regulated autophagy of synaptic vesicles reveals a pathogenic mechanism in motoneuron disease” by Patrick Lüningschrör, Beyenech Binotti, Benjamin Dombert, Peter Heimann, Angel Perez-Lara, Carsten Slotta, Nadine Thau-Habermann, Cora R. von Collenberg, Franziska Karl, Markus Damme, Arie Horowitz, Isabelle Maystadt, Annette Füchtbauer, Ernst-Martin Füchtbauer, Sibylle Jablonka, Robert Blum, Nurcan Üçeyler, Susanne Petri, Barbara Kaltschmidt, Reinhard Jahn, Christian Kaltschmidt & Michael Sendtner in Nature Communications. Published online September 15 2017 doi:10.1038/s41467-017-00689-z






