Summary: Researchers have coupled machine learning with neuroimaging to detect early forms of dementia.
Source: RSNA.
Machine learning is a type of artificial intelligence that allows computer programs to learn when exposed to new data without being programmed. Now, researchers in The Netherlands have coupled machine learning methods with a special MRI technique that measures the perfusion, or tissue absorption rate, of blood throughout the brain to detect early forms of dementia, such as mild cognitive impairment (MCI), according to a new study published online in the journal Radiology.
“MRI can help with the diagnosis of Alzheimer’s disease,” said principal investigator Alle Meije Wink, Ph.D., from the VU University Medical Centre in Amsterdam. “However, the early diagnosis of Alzheimer’s disease is problematic.”
Scientists have long known that Alzheimer’s disease is a gradual process and that the brain undergoes functional changes before the structural changes associated with the disease show up on imaging results. Physicians have no definitive way of identifying who has early dementia or which cases of mild cognitive impairment will progress to Alzheimer’s disease.
“With standard diagnostic MRI, we can see advanced Alzheimer’s disease, such as atrophy of the hippocampus,” Dr. Meije Wink said. “But at that point, the brain tissue is gone and there’s no way to restore it. It would be helpful to detect and diagnose the disease before it’s too late.”
For the new study, the researchers applied machine learning methods to special type of MRI called arterial spin labeling (ASL) imaging. ASL MRI is used to create images called perfusion maps, which show how much blood is delivered to various regions of the brain.
The automated machine learning program is taught to recognize patterns in these maps to distinguish among patients with varying levels of cognitive impairment and predict the stage of Alzheimer’s disease in new (unseen) cases.
The study included 260 of 311 participants from the Alzheimer Center of the VU University Medical Center dementia cohort who underwent ASL MRI between October 2010 and November 2012.
The study group included 100 patients diagnosed with probable Alzheimer’s disease, 60 patients with mild cognitive impairment (MCI) and 100 patients with subjective cognitive decline (SCD), and 26 healthy controls.
SCD and MCI are considered to be early stages of the dementia process and are diagnosed based on the severity of cognitive symptoms, including memory loss and thought- and decision-making problems.
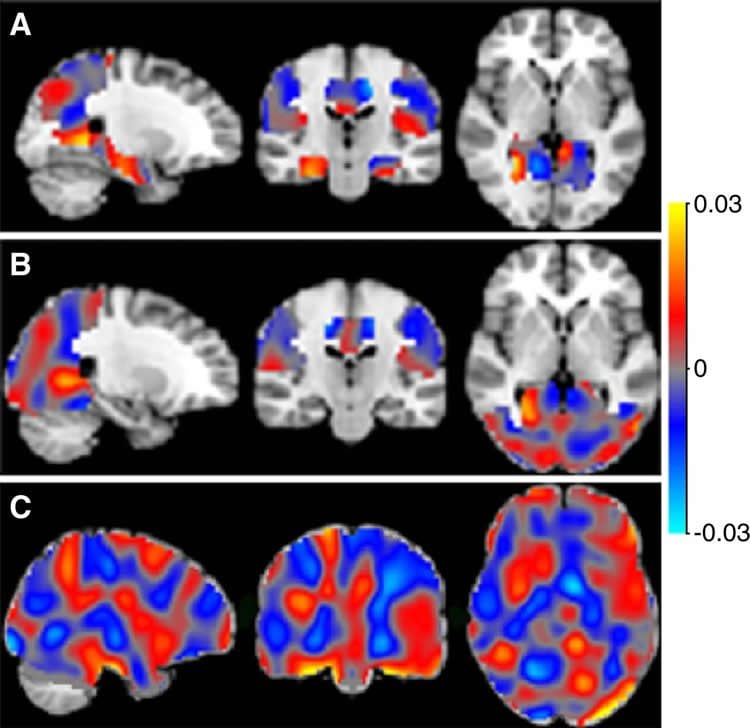
The automated system was able to distinguish effectively among participants with Alzheimer’s disease, MCI and SCD. Using classifiers based on the automated machine learning training, the researchers were then able to predict the Alzheimer’s diagnosis or progression of single patients with a high degree of accuracy, ranging from 82 percent to 90 percent.
“ASL is a promising alternative functional biomarker for the early diagnosis of Alzheimer’s disease,” Dr. Meije Wink said.
He added that the application of automated machine learning methods would be useful as a potential screening tool.
“ASL MRI can identify brain changes that appear early in disease process, when there’s a window of opportunity for intervention,” Dr. Meije Wink said. “If the disease process from SCD to MCI to Alzheimer’s disease could be intercepted or slowed, this technique could play a role in screening.”
Source: Linda Brooks – RSNA
Image Source: This NeuroscienceNews.com image is credited to RSNA.
Original Research: Full open access research for “Application of Machine Learning to Arterial Spin Labeling in Mild Cognitive Impairment and Alzheimer Disease” by Lyduine E. Collij, Fiona Heeman, Joost P. A. Kuijer, Rik Ossenkoppele, Marije R. Benedictus, Christiane Möller, Sander C. J. Verfaillie, Ernesto J. Sanz-Arigita, Bart N. M. van Berckel, Wiesje M. van der Flier, Philip Scheltens, Frederik Barkhof, and Alle Meije Wink in Radiology. Published online July 6 2016 doi:10.1148/radiol.2016152703
[cbtabs][cbtab title=”MLA”]RSNA. “Artificial Intelligence May Aid in Alzheimer’s Diagnosis.” NeuroscienceNews. NeuroscienceNews, 6 July 2016.
<https://neurosciencenews.com/alzheimers-artificial-intelligence-4622/>.[/cbtab][cbtab title=”RSNA”]RSNA. (2016, July 6). Artificial Intelligence May Aid in Alzheimer’s Diagnosis. NeuroscienceNews. Retrieved July 6, 2016 from https://neurosciencenews.com/alzheimers-artificial-intelligence-4622/[/cbtab][cbtab title=”Chicago”]RSNA. “Artificial Intelligence May Aid in Alzheimer’s Diagnosis.” https://neurosciencenews.com/alzheimers-artificial-intelligence-4622/ (accessed July 6, 2016).[/cbtab][/cbtabs]
Abstract
Application of Machine Learning to Arterial Spin Labeling in Mild Cognitive Impairment and Alzheimer Disease
Purpose
To investigate whether multivariate pattern recognition analysis of arterial spin labeling (ASL) perfusion maps can be used for classification and single-subject prediction of patients with Alzheimer disease (AD) and mild cognitive impairment (MCI) and subjects with subjective cognitive decline (SCD) after using the W score method to remove confounding effects of sex and age.
Materials and Methods
Pseudocontinuous 3.0-T ASL images were acquired in 100 patients with probable AD; 60 patients with MCI, of whom 12 remained stable, 12 were converted to a diagnosis of AD, and 36 had no follow-up; 100 subjects with SCD; and 26 healthy control subjects. The AD, MCI, and SCD groups were divided into a sex- and age-matched training set (n = 130) and an independent prediction set (n = 130). Standardized perfusion scores adjusted for age and sex (W scores) were computed per voxel for each participant. Training of a support vector machine classifier was performed with diagnostic status and perfusion maps. Discrimination maps were extracted and used for single-subject classification in the prediction set. Prediction performance was assessed with receiver operating characteristic (ROC) analysis to generate an area under the ROC curve (AUC) and sensitivity and specificity distribution.
Results
Single-subject diagnosis in the prediction set by using the discrimination maps yielded excellent performance for AD versus SCD (AUC, 0.96; P < .01), good performance for AD versus MCI (AUC, 0.89; P < .01), and poor performance for MCI versus SCD (AUC, 0.63; P = .06). Application of the AD versus SCD discrimination map for prediction of MCI subgroups resulted in good performance for patients with MCI diagnosis converted to AD versus subjects with SCD (AUC, 0.84; P < .01) and fair performance for patients with MCI diagnosis converted to AD versus those with stable MCI (AUC, 0.71; P > .05).
Conclusion
With automated methods, age- and sex-adjusted ASL perfusion maps can be used to classify and predict diagnosis of AD, conversion of MCI to AD, stable MCI, and SCD with good to excellent accuracy and AUC values.
“Application of Machine Learning to Arterial Spin Labeling in Mild Cognitive Impairment and Alzheimer Disease” by Lyduine E. Collij, Fiona Heeman, Joost P. A. Kuijer, Rik Ossenkoppele, Marije R. Benedictus, Christiane Möller, Sander C. J. Verfaillie, Ernesto J. Sanz-Arigita, Bart N. M. van Berckel, Wiesje M. van der Flier, Philip Scheltens, Frederik Barkhof, and Alle Meije Wink in Radiology. Published online July 6 2016 doi:10.1148/radiol.2016152703






